当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Substrate Plasticity of a Fungal Peptide α-N-Methyltransferase.
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-06-03 , DOI: 10.1021/acschembio.0c00237 Haigang Song 1, 2 , Ju Ratè Fahrig-Kamarauskaitè 3 , Emmanuel Matabaro 3 , Hannelore Kaspar 3 , Sally L Shirran 4 , Christina Zach 3 , Amy Pace 3 , Bozhidar-Adrian Stefanov 3 , James H Naismith 1, 2, 5 , Markus Künzler 3
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-06-03 , DOI: 10.1021/acschembio.0c00237 Haigang Song 1, 2 , Ju Ratè Fahrig-Kamarauskaitè 3 , Emmanuel Matabaro 3 , Hannelore Kaspar 3 , Sally L Shirran 4 , Christina Zach 3 , Amy Pace 3 , Bozhidar-Adrian Stefanov 3 , James H Naismith 1, 2, 5 , Markus Künzler 3
Affiliation
|
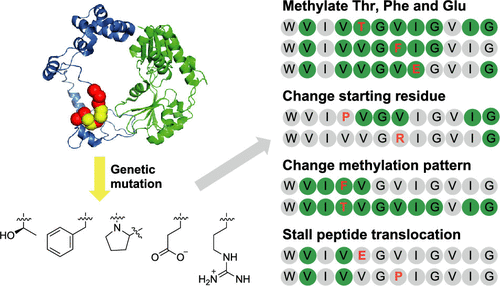
The methylation of amide nitrogen atoms can improve the stability, oral availability, and cell permeability of peptide therapeutics. Chemical N-methylation of peptides is challenging. Omphalotin A is a ribosomally synthesized, macrocylic dodecapeptide with nine backbone N-methylations. The fungal natural product is derived from the precursor protein, OphMA, harboring both the core peptide and a SAM-dependent peptide α-N-methyltransferase domain. OphMA forms a homodimer and its α-N-methyltransferase domain installs the methyl groups in trans on the hydrophobic core dodecapeptide and some additional C-terminal residues of the protomers. These post-translational backbone N-methylations occur in a processive manner from the N- to the C-terminus of the peptide substrate. We demonstrate that OphMA can methylate polar, aromatic, and charged residues when these are introduced into the core peptide. Some of these amino acids alter the efficiency and pattern of methylation. Proline, depending on its sequence context, can act as a tunable stop signal. Crystal structures of OphMA variants have allowed rationalization of these observations. Our results hint at the potential to control this fungal α-N-methyltransferase for biotechnological applications.
中文翻译:

真菌肽α-N-甲基转移酶的底物可塑性。
酰胺氮原子的甲基化可以改善肽治疗剂的稳定性,口服利用率和细胞通透性。肽的化学N-甲基化具有挑战性。卵磷脂A是具有9个主链N-甲基化的核糖体合成的大环十二肽。真菌天然产物衍生自前体蛋白OphMA,它既具有核心肽又具有依赖于SAM的肽α- N-甲基转移酶结构域。OphMA形成同型二聚体,其α- N-甲基转移酶结构域将甲基反式安装在疏水性核心十二肽和前质子的一些其他C末端残基上。这些翻译后主干N-甲基化从肽底物的N-端以C-端进行。我们证明,当将OphMA引入核心肽中时,它们可以甲基化极性,芳香和带电荷的残基。这些氨基酸中的一些改变了甲基化的效率和模式。脯氨酸取决于其序列背景,可以作为可调的终止信号。OphMA变体的晶体结构允许合理化这些观察结果。我们的结果暗示了在生物技术应用中控制这种真菌α- N-甲基转移酶的潜力。
更新日期:2020-07-17
中文翻译:

真菌肽α-N-甲基转移酶的底物可塑性。
酰胺氮原子的甲基化可以改善肽治疗剂的稳定性,口服利用率和细胞通透性。肽的化学N-甲基化具有挑战性。卵磷脂A是具有9个主链N-甲基化的核糖体合成的大环十二肽。真菌天然产物衍生自前体蛋白OphMA,它既具有核心肽又具有依赖于SAM的肽α- N-甲基转移酶结构域。OphMA形成同型二聚体,其α- N-甲基转移酶结构域将甲基反式安装在疏水性核心十二肽和前质子的一些其他C末端残基上。这些翻译后主干N-甲基化从肽底物的N-端以C-端进行。我们证明,当将OphMA引入核心肽中时,它们可以甲基化极性,芳香和带电荷的残基。这些氨基酸中的一些改变了甲基化的效率和模式。脯氨酸取决于其序列背景,可以作为可调的终止信号。OphMA变体的晶体结构允许合理化这些观察结果。我们的结果暗示了在生物技术应用中控制这种真菌α- N-甲基转移酶的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号