当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of Non-covalent Interactions on High-spin Fe(IV)–oxido Complexes
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-06-03 , DOI: 10.1021/jacs.0c03085 Victoria F Oswald 1 , Justin L Lee 1 , Saborni Biswas 2 , Andrew C Weitz 2 , Kaustuv Mittra 3 , Ruixi Fan 2 , Jikun Li 2 , Jiyong Zhao 4 , Michael Y Hu 4 , Esen E Alp 4 , Emile L Bominaar 2 , Yisong Guo 2 , Michael T Green 1, 3 , Michael P Hendrich 2 , A S Borovik 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-06-03 , DOI: 10.1021/jacs.0c03085 Victoria F Oswald 1 , Justin L Lee 1 , Saborni Biswas 2 , Andrew C Weitz 2 , Kaustuv Mittra 3 , Ruixi Fan 2 , Jikun Li 2 , Jiyong Zhao 4 , Michael Y Hu 4 , Esen E Alp 4 , Emile L Bominaar 2 , Yisong Guo 2 , Michael T Green 1, 3 , Michael P Hendrich 2 , A S Borovik 1
Affiliation
|
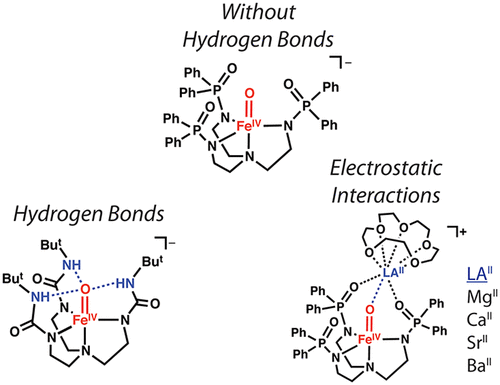
High-valent non-heme FeIV-oxido species are key intermediates in biological oxidation, and their properties are proposed to be influenced by the unique microenvironments present in protein active sites. Microenvironments are regulated by non-covalent interactions, such as hydrogen bonds (H-bonds) and electrostatic interactions; however, there is little quantitative information about how these interactions affect crucial properties of high valent metal-oxido complexes. To address this knowledge gap, we introduced a series of FeIV-oxido complexes that have the same S = 2 spin ground state as those found in nature and then systematically probed the effects of non-covalent interactions on their electronic, structural, and vibrational properties. The key design feature that provides access to these complexes is the new tripodal ligand [poat] 3-, which contains phosphinic amide groups. An important structural aspect of [FeIVpoat(O)] - is the inclusion of an auxiliary site capable of binding a Lewis acid (LAII); we used this unique feature to further modulate the electrostatic environment around the Fe-oxido unit. Experimentally studies confirmed that H-bonds and LAIIs can interact directly with the oxido ligand in FeIV-oxido complexes, which weakens the Fe=O bond and has an impact on the electronic structure. We found that relatively large vibrational changes in the Fe-oxido unit correlate with small structural changes that could be difficult to measure, especially within a protein active site. Our work demonstrates the important role that non-covalent interactions have on the properties of metal complexes; these interactions need to be considered when developing effective oxidants.
中文翻译:

非共价相互作用对高自旋 Fe(IV)-氧化物配合物的影响
高价非血红素 FeIV 氧化物质是生物氧化的关键中间体,它们的性质被认为受蛋白质活性位点中存在的独特微环境的影响。微环境受非共价相互作用的调节,例如氢键(H 键)和静电相互作用;然而,关于这些相互作用如何影响高价金属-氧化物络合物的关键性质的定量信息很少。为了解决这一知识差距,我们引入了一系列与自然界中发现的具有相同 S = 2 自旋基态的 FeIV-氧化配合物,然后系统地探讨了非共价相互作用对其电子、结构和振动特性的影响. 提供对这些复合物的访问的关键设计特征是新的三足配体 [poat] 3-,其中含有次膦酰胺基团。[FeIVpoat(O)] 的一个重要结构方面是包含一个能够结合路易斯酸 (LAII) 的辅助位点;我们使用这个独特的功能来进一步调节铁氧化单元周围的静电环境。实验研究证实,H-键和LAIIs可以直接与FeIV-氧化配合物中的氧化配体相互作用,削弱Fe=O键并对电子结构产生影响。我们发现 Fe-氧化单元中相对较大的振动变化与可能难以测量的微小结构变化相关,尤其是在蛋白质活性位点内。我们的工作证明了非共价相互作用对金属配合物性质的重要作用;在开发有效氧化剂时需要考虑这些相互作用。
更新日期:2020-06-03
中文翻译:

非共价相互作用对高自旋 Fe(IV)-氧化物配合物的影响
高价非血红素 FeIV 氧化物质是生物氧化的关键中间体,它们的性质被认为受蛋白质活性位点中存在的独特微环境的影响。微环境受非共价相互作用的调节,例如氢键(H 键)和静电相互作用;然而,关于这些相互作用如何影响高价金属-氧化物络合物的关键性质的定量信息很少。为了解决这一知识差距,我们引入了一系列与自然界中发现的具有相同 S = 2 自旋基态的 FeIV-氧化配合物,然后系统地探讨了非共价相互作用对其电子、结构和振动特性的影响. 提供对这些复合物的访问的关键设计特征是新的三足配体 [poat] 3-,其中含有次膦酰胺基团。[FeIVpoat(O)] 的一个重要结构方面是包含一个能够结合路易斯酸 (LAII) 的辅助位点;我们使用这个独特的功能来进一步调节铁氧化单元周围的静电环境。实验研究证实,H-键和LAIIs可以直接与FeIV-氧化配合物中的氧化配体相互作用,削弱Fe=O键并对电子结构产生影响。我们发现 Fe-氧化单元中相对较大的振动变化与可能难以测量的微小结构变化相关,尤其是在蛋白质活性位点内。我们的工作证明了非共价相互作用对金属配合物性质的重要作用;在开发有效氧化剂时需要考虑这些相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号