当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modified Fe-Rich Palygorskite as an Efficient and Low-Cost Heterogeneous Fenton Catalyst for NOx and SO2 Removal
Energy & Fuels ( IF 5.3 ) Pub Date : 2020-06-03 , DOI: 10.1021/acs.energyfuels.9b04459 Siyuan Yang 1 , Dan Xu 1 , Shanshan He 1 , Wenjie Yan 1 , Yuanquan Xiong 1
Energy & Fuels ( IF 5.3 ) Pub Date : 2020-06-03 , DOI: 10.1021/acs.energyfuels.9b04459 Siyuan Yang 1 , Dan Xu 1 , Shanshan He 1 , Wenjie Yan 1 , Yuanquan Xiong 1
Affiliation
|
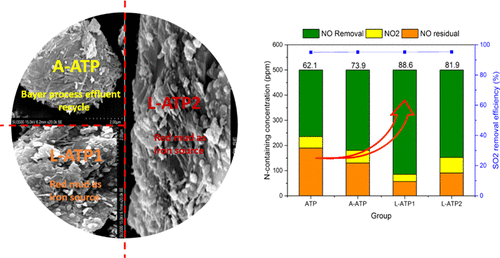
In this article, the entire removal process was based on a cycle absorption system in which a primary-absorber was used to scrub SO2 by urea, then residual NO and SO2 could be oxidized by the vaporized H2O2/catalysts in the catalytic system, and finally, the soluble gaseous pollutants and the remaining gaseous oxidants were absorbed by ammonium sulfite that was produced from the primary-absorber. The complex catalysts formed by iron recovery from the bauxite residue (red mud) were restructured with adenosine triphosphate (ATP) to generate mesopores with surface-active sites (FeOH) for efficient H2O2 catalysis. Further, 95.2% SO2 and 88.6% NO were removed from flue gas under the operation conditions where SO2 concentration was 2000 ppm, NO concentration was 500 ppm, O2 concentration was 7%, CO2 concentration was 10%, catalytic temperature was 140 °C, H2O2 feeding rate was 1.5 mL/h, and the total gas flow rate was 1.5 L/min. The results suggested that the catalytic performance is closely related to the structure of support ATP, which was elucidated by a series of experiments in different Fe loadings and BET characterization. From electron spin resonance (ESR) results, H2O2 generally decompose not only into the •OH free radical by iron oxide but also into •HOO and related reactive oxygen species. According to the results of X-ray photoelectron spectroscopy (XPS) and temperature-programmed reduction (TPR), the content of FeOH and lattice oxygen in the modified ATP catalyst played an important role in the adsorption of H2O2 on the protonated surface of the low-acid catalyst. Furthermore, the rational catalytic mechanism of catalyst/H2O2 at low and high temperatures (corresponding to 140 and 200 °C) was proposed by transient response experiments.
中文翻译:

改性富Fe坡缕石作为一种高效且低成本的异构芬顿催化剂NO X和SO 2去除
在本文中,整个去除过程基于循环吸收系统,在该系统中,主要吸收器用于通过尿素洗涤SO 2,然后残留的NO和SO 2会被H 2 O 2 /催化剂中的气化催化剂氧化。催化系统,最后,可溶性气态污染物和剩余的气态氧化剂被一次吸收器产生的亚硫酸铵吸收。通过从铝土矿残渣(红泥)中回收铁而形成的复合催化剂用三磷酸腺苷(ATP)进行重组,以生成具有表面活性中心(FeOH)的中孔,从而有效地进行H 2 O 2催化。此外,SO 2 95.2%在SO 2浓度为2000 ppm,NO浓度为500 ppm,O 2浓度为7%,CO 2浓度为10%,催化温度为140°C,H 2的操作条件下,从烟道气中除去了88.6%的NO。O 2进料速度为1.5 mL / h,总气体流速为1.5 L / min。结果表明,催化性能与载体ATP的结构密切相关,这通过在不同的Fe含量和BET表征条件下进行的一系列实验得以阐明。根据电子自旋共振(ESR)结果,H 2 O 2通常不仅会被氧化铁分解为• OH自由基,而且还会分解为• HOO和相关的活性氧种类。根据X射线光电子能谱(XPS)和程序升温还原(TPR)的结果,改性ATP催化剂中FeOH和晶格氧的含量在质子化表面吸附H 2 O 2方面起着重要作用。低酸催化剂。此外,催化剂的合理催化机制/ H 2 ö 2在低温和高温下(相应于140和200℃)提出的瞬态响应实验。
更新日期:2020-07-16
中文翻译:

改性富Fe坡缕石作为一种高效且低成本的异构芬顿催化剂NO X和SO 2去除
在本文中,整个去除过程基于循环吸收系统,在该系统中,主要吸收器用于通过尿素洗涤SO 2,然后残留的NO和SO 2会被H 2 O 2 /催化剂中的气化催化剂氧化。催化系统,最后,可溶性气态污染物和剩余的气态氧化剂被一次吸收器产生的亚硫酸铵吸收。通过从铝土矿残渣(红泥)中回收铁而形成的复合催化剂用三磷酸腺苷(ATP)进行重组,以生成具有表面活性中心(FeOH)的中孔,从而有效地进行H 2 O 2催化。此外,SO 2 95.2%在SO 2浓度为2000 ppm,NO浓度为500 ppm,O 2浓度为7%,CO 2浓度为10%,催化温度为140°C,H 2的操作条件下,从烟道气中除去了88.6%的NO。O 2进料速度为1.5 mL / h,总气体流速为1.5 L / min。结果表明,催化性能与载体ATP的结构密切相关,这通过在不同的Fe含量和BET表征条件下进行的一系列实验得以阐明。根据电子自旋共振(ESR)结果,H 2 O 2通常不仅会被氧化铁分解为• OH自由基,而且还会分解为• HOO和相关的活性氧种类。根据X射线光电子能谱(XPS)和程序升温还原(TPR)的结果,改性ATP催化剂中FeOH和晶格氧的含量在质子化表面吸附H 2 O 2方面起着重要作用。低酸催化剂。此外,催化剂的合理催化机制/ H 2 ö 2在低温和高温下(相应于140和200℃)提出的瞬态响应实验。


























 京公网安备 11010802027423号
京公网安备 11010802027423号