当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Methionine-Rich Loop of Multicopper Oxidase McoA Follows Open-to-Close Transitions with a Role in Enzyme Catalysis
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-06-02 , DOI: 10.1021/acscatal.0c01623 Patrícia T. Borges, Vânia Brissos, Guillem Hernandez, Laura Masgrau, Maria Fátima Lucas, Emanuele Monza, Carlos Frazão, Tiago N. Cordeiro, Lígia O. Martins
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-06-02 , DOI: 10.1021/acscatal.0c01623 Patrícia T. Borges, Vânia Brissos, Guillem Hernandez, Laura Masgrau, Maria Fátima Lucas, Emanuele Monza, Carlos Frazão, Tiago N. Cordeiro, Lígia O. Martins
|
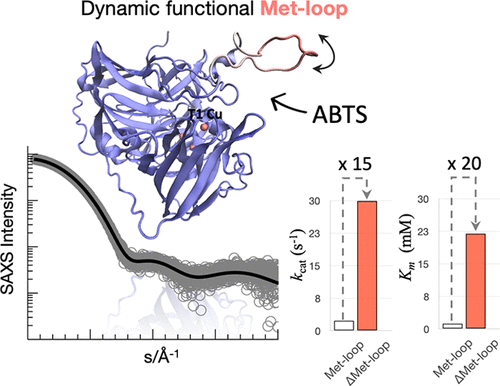
Multicopper oxidases oxidize a vast range of aromatic substrates coupled to the reduction of molecular oxygen to water. A vast broad spectrum of applications reflects their high biotechnological importance. The crystal structure of McoA from the hyperthermophilic bacteria Aquifex aeolicus has the most tightly compact and hydrophobic core among its prokaryotic counterparts. A 29-residue long loop enriched in glycines and methionines (Met-loop) close to the active T1 Cu center is not detected in the electron density maps. Accurate prediction of loop structures remains challenging, especially for long segments with sizable conformational space. Therefore, a combination of Rosetta and molecular dynamics simulations with ensemble-based small-angle X-ray scattering analysis was used to probe the conformational landscape of the Met-loop. The results indicate a highly flexible omega-loop, which is nevertheless not random but preferentially follows open-to-close transitions, exposing or occluding the T1 Cu site. Loop-truncated variants maintain wild-type stability and consistently lower and higher catalytic efficiencies (kcat/Km) for organic and metal substrates, respectively. Our results suggest that the loop transient dynamic equilibrium can exert important switch-like regulatory function, defining a role for Met-rich motifs as dynamic gate-gappers. This work provides insights into the dynamics of Met-rich loops essential to understand the molecular determinants of substrate promiscuity and catalytic rates within multicopper oxidases. We anticipate that engineering the Met-loop structural dynamics will unleash important changes in enzyme function and specificity with impact on their applications.
中文翻译:

富含铜的氧化酶McoA的蛋氨酸富集环跟随从开到关的转变,在酶催化中起作用
Multicopper氧化酶可氧化多种芳香族底物,从而将分子氧还原为水。广泛的应用范围反映了其高度的生物技术重要性。嗜热菌Aquifex aeolicus的McoA的晶体结构在原核生物中具有最紧密和疏水的核心。在电子密度图中未检测到29个残基的长环,富含甘氨酸和蛋氨酸(Met环),靠近活性T1 Cu中心。对环结构的准确预测仍然具有挑战性,尤其是对于具有相当大的构象空间的长链段。因此,将Rosetta和分子动力学模拟与基于整体的小角度X射线散射分析相结合,用于探测Met环的构象态势。结果表明高度灵活的欧米茄环,尽管它不是随机的,但优先遵循开闭转换,从而暴露或闭塞T1 Cu位点。环截短的变体保持野生型稳定性,并始终具有较低和较高的催化效率(k cat / K m)分别用于有机和金属基材。我们的结果表明,环路瞬态动态平衡可以发挥重要的开关样调节功能,从而定义了丰富的Met图案作为动态门间隙的作用。这项工作提供了深入了解Met富集环动力学的见解,这些动力学对于理解底物混杂分子决定因素和多铜氧化酶中的催化速率至关重要。我们预计,工程化的Met环结构动力学将释放酶功能和特异性的重要变化,从而影响其应用。
更新日期:2020-07-02
中文翻译:

富含铜的氧化酶McoA的蛋氨酸富集环跟随从开到关的转变,在酶催化中起作用
Multicopper氧化酶可氧化多种芳香族底物,从而将分子氧还原为水。广泛的应用范围反映了其高度的生物技术重要性。嗜热菌Aquifex aeolicus的McoA的晶体结构在原核生物中具有最紧密和疏水的核心。在电子密度图中未检测到29个残基的长环,富含甘氨酸和蛋氨酸(Met环),靠近活性T1 Cu中心。对环结构的准确预测仍然具有挑战性,尤其是对于具有相当大的构象空间的长链段。因此,将Rosetta和分子动力学模拟与基于整体的小角度X射线散射分析相结合,用于探测Met环的构象态势。结果表明高度灵活的欧米茄环,尽管它不是随机的,但优先遵循开闭转换,从而暴露或闭塞T1 Cu位点。环截短的变体保持野生型稳定性,并始终具有较低和较高的催化效率(k cat / K m)分别用于有机和金属基材。我们的结果表明,环路瞬态动态平衡可以发挥重要的开关样调节功能,从而定义了丰富的Met图案作为动态门间隙的作用。这项工作提供了深入了解Met富集环动力学的见解,这些动力学对于理解底物混杂分子决定因素和多铜氧化酶中的催化速率至关重要。我们预计,工程化的Met环结构动力学将释放酶功能和特异性的重要变化,从而影响其应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号