当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Roles of Lewis Acid Catalysts in Diels-Alder Reactions between Cyclopentadiene and Methyl Acrylate.
ChemistryOpen ( IF 2.3 ) Pub Date : 2020-06-03 , DOI: 10.1002/open.202000112 Ken Sakata 1 , Hiroshi Fujimoto 2
ChemistryOpen ( IF 2.3 ) Pub Date : 2020-06-03 , DOI: 10.1002/open.202000112 Ken Sakata 1 , Hiroshi Fujimoto 2
Affiliation
|
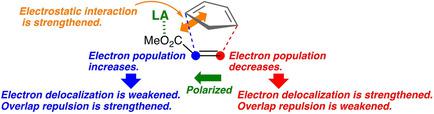
The Diels‐Alder reaction of cyclopentadiene with methyl acrylate catalyzed by AlCl3 has been theoretically investigated. M06‐2X level DFT calculations have shown that the formation of two C−C bonds is asynchronous in the cycloaddition both in the endo path and in the exo path, thus making a good contrast to the well‐known concept of [4+2] reactions based on the orbital symmetry arguments. It was found that the catalyst facilitates the cycloaddition and brings a higher endo selectivity in the highly asynchronous process, as compared with the reaction of the diene and the dienophile without the catalyst.
中文翻译:

Lewis酸催化剂在环戊二烯与丙烯酸甲酯之间的Diels-Alder反应中的作用。
理论上研究了环戊二烯与AlCl 3催化的丙烯酸甲酯的Diels-Alder反应。M06-2X水平DFT计算已经表明,两个C-C键的形成是在无论是在环加成异步内路径和外路径,从而使良好的对比[4 + 2]公知的概念基于轨道对称性参数的反应。与没有催化剂的二烯和亲二烯体的反应相比,发现该催化剂在高度异步的过程中促进环加成并带来更高的内选择性。
更新日期:2020-06-03
中文翻译:

Lewis酸催化剂在环戊二烯与丙烯酸甲酯之间的Diels-Alder反应中的作用。
理论上研究了环戊二烯与AlCl 3催化的丙烯酸甲酯的Diels-Alder反应。M06-2X水平DFT计算已经表明,两个C-C键的形成是在无论是在环加成异步内路径和外路径,从而使良好的对比[4 + 2]公知的概念基于轨道对称性参数的反应。与没有催化剂的二烯和亲二烯体的反应相比,发现该催化剂在高度异步的过程中促进环加成并带来更高的内选择性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号