Biochimica et Biophysica Acta (BBA) - Molecular Cell Research ( IF 5.1 ) Pub Date : 2020-06-02 , DOI: 10.1016/j.bbamcr.2020.118765 Liqun Yu 1 , Lawrence Wang 1 , Ji Eun Kim 1 , Chengjian Mao 1 , David J Shapiro 2
|
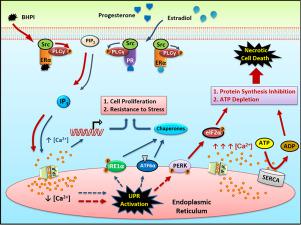
Accumulation of unfolded protein, or other stresses, activates the classical reactive unfolded protein response (UPR). In the recently characterized anticipatory UPR, receptor-bound estrogen, progesterone and other mitogenic hormones rapidly elicit phosphorylation of phospholipase C γ (PLCγ), activating the anticipatory UPR. How estrogen and progesterone activating their receptors couples to PLCγ phosphorylation and anticipatory UPR activation was unknown. We show that the oncogene c-Src is a rate-limiting regulator whose tyrosine kinase activity links estrogen and progesterone activating their receptors to anticipatory UPR activation. Supporting Src coupling estrogen and progesterone to anticipatory UPR activation, we identified extranuclear complexes of estrogen receptor α (ERα):Src:PLCγ and progesterone receptor:Src:PLCγ. Moreover, Src inhibition protected cancer cells against cell death. To probe Src's role, we used the preclinical ERα biomodulator, BHPI, which kills cancer cells by inducing lethal anticipatory UPR hyperactivation. Notably, Src inhibition blocked BHPI-mediated anticipatory UPR activation and the resulting rapid increase in intracellular calcium. After unbiased long-term selection for BHPI-resistant human breast cancer cells, 4/11 BHPI-resistant T47D clones, and nearly all MCF-7 clones, exhibited reduced levels of normally growth-stimulating Src. Notably, Src overexpression by virus transduction restored sensitivity to BHPI. Furthermore, in wild type cells, several-fold knockdown of Src, but not of ERα, strongly blocked BHPI-mediated UPR activation and subsequent HMGB1 release and necrotic cell death. Thus, Src plays a previously undescribed pivotal role in activation of the tumor-protective anticipatory UPR, thereby increasing the resilience of breast cancer cells. This is a new role for Src and the anticipatory UPR in breast cancer.
中文翻译:

Src 将雌激素受体与预期的未折叠蛋白反应结合,并在压力下调节癌细胞的命运。
未折叠蛋白的积累或其他压力会激活经典的反应性未折叠蛋白反应 (UPR)。在最近表征的预期 UPR 中,受体结合的雌激素、孕酮和其他促有丝分裂激素迅速引发磷脂酶 C γ (PLCγ) 的磷酸化,激活预期 UPR。雌激素和孕激素如何激活它们的受体与 PLCγ 磷酸化和预期的 UPR 激活结合尚不清楚。我们表明致癌基因 c-Src 是一种限速调节剂,其酪氨酸激酶活性将雌激素和孕酮激活其受体与预期的 UPR 激活联系起来。支持 Src 将雌激素和孕酮与预期的 UPR 激活偶联,我们鉴定了雌激素受体 α (ERα):Src:PLCγ 和孕酮受体:Src:PLCγ 的核外复合物。而且,Src 抑制保护癌细胞免受细胞死亡。为了探究 Src 的作用,我们使用了临床前 ERα 生物调节剂 BHPI,它通过诱导致命的预期 UPR 过度激活来杀死癌细胞。值得注意的是,Src 抑制阻止了 BHPI 介导的预期 UPR 激活和由此导致的细胞内钙的快速增加。在对 BHPI 抗性人乳腺癌细胞进行无偏见的长期选择后,4/11 BHPI 抗性 T47D 克隆和几乎所有 MCF-7 克隆表现出正常生长刺激 Src 的水平降低。值得注意的是,病毒转导导致的 Src 过表达恢复了对 BHPI 的敏感性。此外,在野生型细胞中,Src 而非 ERα 的数倍敲低强烈阻止了 BHPI 介导的 UPR 激活和随后的 HMGB1 释放和坏死细胞死亡。因此,Src 在激活肿瘤保护性预期 UPR 中发挥了以前未描述的关键作用,从而增加了乳腺癌细胞的恢复能力。这是 Src 和预期 UPR 在乳腺癌中的新作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号