当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical regioselective alkylations of a [60]fulleroindoline with bulky alkyl bromides.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-06-02 , DOI: 10.1039/d0ob00876a Yong Yang 1 , Chuang Niu 1 , Muqing Chen 2 , Shangfeng Yang 2 , Guan-Wu Wang 3
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-06-02 , DOI: 10.1039/d0ob00876a Yong Yang 1 , Chuang Niu 1 , Muqing Chen 2 , Shangfeng Yang 2 , Guan-Wu Wang 3
Affiliation
|
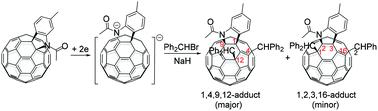
Electrochemical alkylations of a [60]fulleroindoline with different bulky alkyl bromides exhibit different reaction behaviors. The hydroalkylation and dialkylation of the electrochemically generated dianionic [60]fulleroindoline with bulky 2,4,6-tris(bromomethyl)mesitylene give rise to 1,2,3,16-adducts. In comparison, the hydroalkylation of the dianionic [60]fulleroindoline with bulkier diphenylbromomethane still affords a 1,2,3,16-adduct, while the corresponding dialkylation provides a sterically favoured 1,4,9,12-adduct, which is scarcely investigated, as the major product along with the isomeric 1,2,3,16-adduct as the minor product. The structures of these products have been determined by spectroscopic data and single-crystal X-ray diffraction analysis. A plausible reaction mechanism has been proposed to explain the formation of the observed products.
中文翻译:

[60]富勒吲哚啉与大体积烷基溴的电化学区域选择性烷基化。
[60]富勒吲哚啉与不同的大体积烷基溴的电化学烷基化表现出不同的反应行为。电化学生成的双阴离子[60]富二氢吲哚与笨重的2,4,6-三(溴甲基)亚甲基苯的加氢烷基化和二烷基化反应产生1,2,3,16加合物。相比之下,双阴离子[60]富勒吲哚啉与体积较大的二苯基溴甲烷的加氢烷基化反应仍可得到1,2,3,16加合物,而相应的二烷基化反应可得到空间上有利的1,4,9,12加合物,对此很少进行研究。 ,作为主要产物,同分异构的1,2,3,16加合物作为次要产物。这些产品的结构已通过光谱数据和单晶X射线衍射分析确定。
更新日期:2020-07-01
中文翻译:

[60]富勒吲哚啉与大体积烷基溴的电化学区域选择性烷基化。
[60]富勒吲哚啉与不同的大体积烷基溴的电化学烷基化表现出不同的反应行为。电化学生成的双阴离子[60]富二氢吲哚与笨重的2,4,6-三(溴甲基)亚甲基苯的加氢烷基化和二烷基化反应产生1,2,3,16加合物。相比之下,双阴离子[60]富勒吲哚啉与体积较大的二苯基溴甲烷的加氢烷基化反应仍可得到1,2,3,16加合物,而相应的二烷基化反应可得到空间上有利的1,4,9,12加合物,对此很少进行研究。 ,作为主要产物,同分异构的1,2,3,16加合物作为次要产物。这些产品的结构已通过光谱数据和单晶X射线衍射分析确定。


























 京公网安备 11010802027423号
京公网安备 11010802027423号