当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Control of transition metal–oxygen bond strength boosts the redox ex-solution in a perovskite oxide surface
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2020-06-02 , DOI: 10.1039/d0ee01308k Kyeounghak Kim 1, 2, 3, 4 , Bonjae Koo 4, 5, 6, 7 , Yong-Ryun Jo 4, 8, 9, 10 , Siwon Lee 4, 5, 6, 7 , Jun Kyu Kim 4, 5, 6, 7 , Bong-Joong Kim 4, 8, 9, 10 , WooChul Jung 4, 5, 6, 7 , Jeong Woo Han 1, 2, 3, 4
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2020-06-02 , DOI: 10.1039/d0ee01308k Kyeounghak Kim 1, 2, 3, 4 , Bonjae Koo 4, 5, 6, 7 , Yong-Ryun Jo 4, 8, 9, 10 , Siwon Lee 4, 5, 6, 7 , Jun Kyu Kim 4, 5, 6, 7 , Bong-Joong Kim 4, 8, 9, 10 , WooChul Jung 4, 5, 6, 7 , Jeong Woo Han 1, 2, 3, 4
Affiliation
|
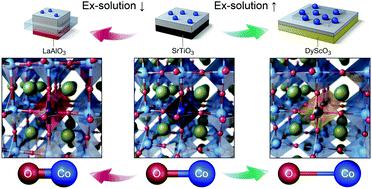
We demonstrate theoretically and experimentally that engineering of cation–oxygen bond strength in a perovskite structure can control redox ex-solution of B-site metals and thus the formation of metal nanoparticles at the oxide surface upon high-temperature reduction. In particular, we show that large isovalent doping significantly promotes the B-site ex-solution via tuning of the cation–oxygen bond strength, leading to high catalytic activity of CO oxidation. This method to promote ex-solution can be readily applied to various heterogeneous catalysts.
中文翻译:

控制过渡金属与氧的结合强度可增强钙钛矿氧化物表面的氧化还原溶液。
我们从理论和实验上证明,钙钛矿结构中阳离子-氧键强度的工程化可以控制B位金属的氧化还原析出,因此可以控制高温还原后在氧化物表面形成金属纳米粒子。特别是,我们表明,通过调节阳离子-氧键的强度,大等价掺杂可以显着促进B位解离,从而导致CO氧化的高催化活性。该促进解溶液的方法可以容易地应用于各种非均相催化剂。
更新日期:2020-06-02
中文翻译:

控制过渡金属与氧的结合强度可增强钙钛矿氧化物表面的氧化还原溶液。
我们从理论和实验上证明,钙钛矿结构中阳离子-氧键强度的工程化可以控制B位金属的氧化还原析出,因此可以控制高温还原后在氧化物表面形成金属纳米粒子。特别是,我们表明,通过调节阳离子-氧键的强度,大等价掺杂可以显着促进B位解离,从而导致CO氧化的高催化活性。该促进解溶液的方法可以容易地应用于各种非均相催化剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号