当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sustainable Straightforward Synthesis and Evaluation of the Antioxidant and Antimicrobial Activity of Sinapine and Analogues.
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2020-06-02 , DOI: 10.1021/acs.jafc.0c02183 Louis M M Mouterde 1 , Aurélien A M Peru 1 , Matthieu M Mention 1 , Fanny Brunissen 1 , Florent Allais 1
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2020-06-02 , DOI: 10.1021/acs.jafc.0c02183 Louis M M Mouterde 1 , Aurélien A M Peru 1 , Matthieu M Mention 1 , Fanny Brunissen 1 , Florent Allais 1
Affiliation
|
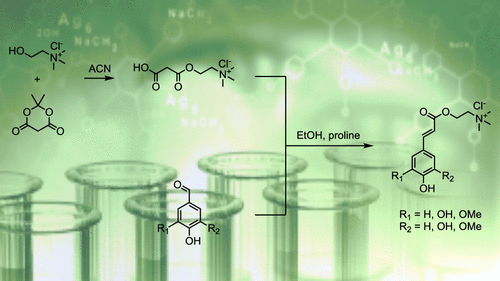
Naturally occurring sinapine was successfully synthesized through a proline-mediated Knoevenagel–Doebner condensation in ethanol. This synthetic process involving biobased syringaldehyde, Meldrum’s acid, and choline chloride offers a sustainable alternative to the existing low-yield pathways. This two-step strategy gives access to sinapine in a 52% overall yield and has been implemented in the synthesis of sinapine analogues, using 4-hydroxybenzaldehyde, 3,4-dihydroxybenzaldehyde, and vanillin as precursors, giving target molecules with 34–61% overall isolated yields. The purity of synthetic sinapine and its analogues (ca. 95%) was assessed by NMR and high-performance liquid chromatography—mass spectrometry analyses. Furthermore, the antioxidant and antimicrobial activities were assessed, and the potential of this series of molecules was confirmed.
中文翻译:

可持续的简单合成方法以及对芥子油及其类似物的抗氧化和抗菌活性的评估。
通过脯氨酸介导的Knoevenagel-Doebner在乙醇中的缩合成功合成了天然的西那平。这种涉及生物基丁香醛,梅德鲁姆酸和氯化胆碱的合成方法为现有的低产率途径提供了可持续的替代方法。此两步策略使辛那平的总收率达到52%,并且已在以4-羟基苯甲醛,3,4-二羟基苯甲醛和香草醛为前体的辛纳平类似物的合成中实施,目标分子的产率为34-61%整体孤立的产量。合成芥子碱的纯度及其类似物(约95%)通过NMR和高效液相色谱-质谱分析进行评估。此外,评估了抗氧化剂和抗菌活性,并证实了这一系列分子的潜力。
更新日期:2020-07-01
中文翻译:

可持续的简单合成方法以及对芥子油及其类似物的抗氧化和抗菌活性的评估。
通过脯氨酸介导的Knoevenagel-Doebner在乙醇中的缩合成功合成了天然的西那平。这种涉及生物基丁香醛,梅德鲁姆酸和氯化胆碱的合成方法为现有的低产率途径提供了可持续的替代方法。此两步策略使辛那平的总收率达到52%,并且已在以4-羟基苯甲醛,3,4-二羟基苯甲醛和香草醛为前体的辛纳平类似物的合成中实施,目标分子的产率为34-61%整体孤立的产量。合成芥子碱的纯度及其类似物(约95%)通过NMR和高效液相色谱-质谱分析进行评估。此外,评估了抗氧化剂和抗菌活性,并证实了这一系列分子的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号