当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multifunctional Core-Shell Glyconanoparticles for Galectin-3-Targeted, Trigger-Responsive Combination Chemotherapy.
Biomacromolecules ( IF 6.2 ) Pub Date : 2020-06-02 , DOI: 10.1021/acs.biomac.0c00358 Biji Balakrishnan 1 , Suresh Subramanian 2, 3 , Madhava B Mallia 2, 3 , Krishnamohan Repaka 4 , Shahdeep Kaur 1 , Rajeet Chandan 1 , Prateek Bhardwaj 1 , Ashutosh Dash 2, 3 , Rinti Banerjee 1
Biomacromolecules ( IF 6.2 ) Pub Date : 2020-06-02 , DOI: 10.1021/acs.biomac.0c00358 Biji Balakrishnan 1 , Suresh Subramanian 2, 3 , Madhava B Mallia 2, 3 , Krishnamohan Repaka 4 , Shahdeep Kaur 1 , Rajeet Chandan 1 , Prateek Bhardwaj 1 , Ashutosh Dash 2, 3 , Rinti Banerjee 1
Affiliation
|
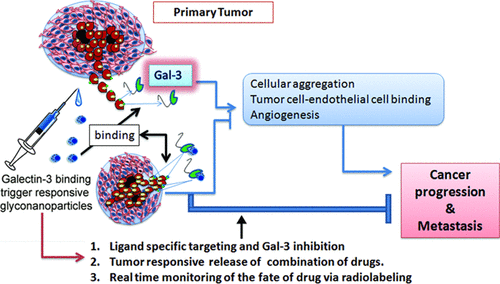
Galectin-3 (gal-3) plays a crucial role in various cellular events associated to tumor metastasis and progression. In this direction, gal-3 binding core–shell glyconanoparticles based on citrus pectin (CP) have been designed for targeted, trigger-responsive combination drug delivery. Depolymerization via periodate oxidation in heterogeneous medium yielded low-molecular weight dialdehyde oligomers (CPDA) of CP with a gal-3 binding property (Kd = 160.90 μM). CPDA-based core–shell nanoparticles prepared to enhance the gal-3 binding specificity via a multivalent ligand presentation have shown to reduce homotypic cellular aggregation, tumor cell binding with endothelial cells, and endothelial tube formation, the major steps involved in the progression of cancer. Immune-fluorescence and flow cytometric analysis confirmed significant reduction in gal-3 expression on MDA-MB 231 cancer cells upon incubation with nanoparticles. An on-demand tumor microenvironment-responsive release of drugs at low pH and high concentrations of glucose and glutathione prevailing in tumor milieu was achieved by introducing a cleavable Schiff’s base, a boronate ester, and disulfide linkages within the shell of the nanoparticles. Nanoparticles with encapsulated sulindac in the core and doxorubicin (DOX) in the shell demonstrated target specificity and enhanced internalization with synergistic cytotoxic effects with a 30-fold reduction in IC50 in DOX-resistant, triple-negative MDA-MB 231 breast cancer cells. Nanoparticles were radiolabeled with 131I radioisotopes with ≥80% efficiency while retaining its gal-3 binding property. Biodistribution studies of radiolabeled placebo nanoparticles and drug-loaded CPDA nanoparticles demonstrated proof of concept of gal-3 targeting seen as preferential accumulation in the gal-3-expressing tissues of the gastric tract. The CPDA core–shell nanoparticles are thus promising platforms for gal-3 targeting and inhibition of gal-3-mediated processes involved in cancer progression with a potential of radiolabeling for in vivo monitoring or delivering therapeutic doses of radiation and on-demand triggered, target-specific drug release.
中文翻译:

用于Galectin-3靶向,触发反应的联合化疗的多功能核壳糖醇纳米颗粒。
Galectin-3(gal-3)在与肿瘤转移和进展相关的各种细胞事件中起着至关重要的作用。在这个方向上,基于柑桔果胶(CP)的gal-3结合核-壳糖纳米颗粒已被设计用于靶向的,触发反应的组合药物递送。在异质介质中通过高碘酸氧化解聚反应得到具有gal-3结合特性的CP的低分子量二醛低聚物(CPDA)(K d= 160.90μM)。基于CPDA的核壳纳米颗粒通过多价配体的表达增强gal-3结合的特异性,可以减少同型细胞聚集,肿瘤细胞与内皮细胞的结合以及内皮管的形成,这是癌症进展的主要步骤。免疫荧光和流式细胞仪分析证实,与纳米颗粒孵育后,MDA-MB 231癌细胞上gal-3表达显着降低。通过在纳米颗粒的壳层中引入可裂解的席夫碱,硼酸酯和二硫键,可以实现在低pH值以及在肿瘤环境中普遍存在的高浓度葡萄糖和谷胱甘肽的按需肿瘤微环境响应性药物释放。50个抗DOX的三阴性MDA-MB 231乳腺癌细胞。用131 I放射性同位素对纳米粒子进行放射性标记,效率≥80%,同时保留其gal-3结合特性。放射性标记的安慰剂纳米颗粒和载有药物的CPDA纳米颗粒的生物分布研究证明了gal-3靶向概念的证明,该概念被视为在胃道gal-3表达组织中的优先积累。因此,CPDA核-壳纳米粒子是有希望的平台,可用于靶向gal-3靶向和抑制与癌症进展有关的gal-3介导的过程,并具有放射性标记的潜力,可用于体内监测或提供治疗剂量的放射和按需触发的靶标特异性药物释放。
更新日期:2020-07-13
中文翻译:

用于Galectin-3靶向,触发反应的联合化疗的多功能核壳糖醇纳米颗粒。
Galectin-3(gal-3)在与肿瘤转移和进展相关的各种细胞事件中起着至关重要的作用。在这个方向上,基于柑桔果胶(CP)的gal-3结合核-壳糖纳米颗粒已被设计用于靶向的,触发反应的组合药物递送。在异质介质中通过高碘酸氧化解聚反应得到具有gal-3结合特性的CP的低分子量二醛低聚物(CPDA)(K d= 160.90μM)。基于CPDA的核壳纳米颗粒通过多价配体的表达增强gal-3结合的特异性,可以减少同型细胞聚集,肿瘤细胞与内皮细胞的结合以及内皮管的形成,这是癌症进展的主要步骤。免疫荧光和流式细胞仪分析证实,与纳米颗粒孵育后,MDA-MB 231癌细胞上gal-3表达显着降低。通过在纳米颗粒的壳层中引入可裂解的席夫碱,硼酸酯和二硫键,可以实现在低pH值以及在肿瘤环境中普遍存在的高浓度葡萄糖和谷胱甘肽的按需肿瘤微环境响应性药物释放。50个抗DOX的三阴性MDA-MB 231乳腺癌细胞。用131 I放射性同位素对纳米粒子进行放射性标记,效率≥80%,同时保留其gal-3结合特性。放射性标记的安慰剂纳米颗粒和载有药物的CPDA纳米颗粒的生物分布研究证明了gal-3靶向概念的证明,该概念被视为在胃道gal-3表达组织中的优先积累。因此,CPDA核-壳纳米粒子是有希望的平台,可用于靶向gal-3靶向和抑制与癌症进展有关的gal-3介导的过程,并具有放射性标记的潜力,可用于体内监测或提供治疗剂量的放射和按需触发的靶标特异性药物释放。



























 京公网安备 11010802027423号
京公网安备 11010802027423号