当前位置:
X-MOL 学术
›
Isr. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Update on Distal C(sp3)−H Functionalization Involving 1,5‐HAT Emerging from Nitrogen Radicals
Israel Journal of Chemistry ( IF 3.2 ) Pub Date : 2020-03-23 , DOI: 10.1002/ijch.201900172 Nupur Goswami 1 , Debabrata Maiti 1
Israel Journal of Chemistry ( IF 3.2 ) Pub Date : 2020-03-23 , DOI: 10.1002/ijch.201900172 Nupur Goswami 1 , Debabrata Maiti 1
Affiliation
|
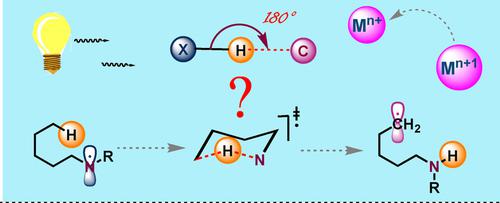
Methods to selectively functionalize any one sp3 C−H bond among all others, has been well documented in literature. Radical reactions, which are essentially mild reaction conditions has provided a significant improvement over the standard functionalization pathways. Although radical recombinations are fast and feasible, the selectivity is always guided by the electronic biasness in the system. 1,n‐Hydrogen atom transfer (HAT) reactions are extremely useful in determining regioselectivity, the involvement of a 1,5‐HAT protocol made the reaction pathway energetically much more favourable to functionalize the desired remote C(sp3)−H bond. In this review we are going to give a brief overview of the methods involved in the functionalization of distal aliphatic C−H bond by 1,5‐HAT transformation pathway.
中文翻译:

涉及源自氮自由基的1,5-HAT的远端C(sp3)-H功能化的更新
选择性地功能化任何一个sp 3 C-H键以及其他键的方法,在文献中已有充分的文献记载。基本上是温和的反应条件的自由基反应已经对标准功能化途径进行了重大改进。尽管自由基复合是快速且可行的,但选择性始终受系统中电子偏压的影响。1,n-氢原子转移(HAT)反应在确定区域选择性方面非常有用,1,5-HAT方案的参与使反应途径在能量上更有利于功能化所需的远程C(sp 3)-H键。在这篇综述中,我们将简要概述通过1,5-HAT转化途径对远端脂肪族CH键进行功能化的方法。
更新日期:2020-03-23
中文翻译:

涉及源自氮自由基的1,5-HAT的远端C(sp3)-H功能化的更新
选择性地功能化任何一个sp 3 C-H键以及其他键的方法,在文献中已有充分的文献记载。基本上是温和的反应条件的自由基反应已经对标准功能化途径进行了重大改进。尽管自由基复合是快速且可行的,但选择性始终受系统中电子偏压的影响。1,n-氢原子转移(HAT)反应在确定区域选择性方面非常有用,1,5-HAT方案的参与使反应途径在能量上更有利于功能化所需的远程C(sp 3)-H键。在这篇综述中,我们将简要概述通过1,5-HAT转化途径对远端脂肪族CH键进行功能化的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号