当前位置:
X-MOL 学术
›
Isr. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular Dynamics Simulations of Antibiotic Ceftaroline at the Allosteric Site of Penicillin‐Binding Protein 2a (PBP2a)
Israel Journal of Chemistry ( IF 3.2 ) Pub Date : 2020-04-16 , DOI: 10.1002/ijch.202000012 Ying‐Chih Chiang 1 , Mabel T. Y. Wong 1 , Jonathan W. Essex 1
Israel Journal of Chemistry ( IF 3.2 ) Pub Date : 2020-04-16 , DOI: 10.1002/ijch.202000012 Ying‐Chih Chiang 1 , Mabel T. Y. Wong 1 , Jonathan W. Essex 1
Affiliation
|
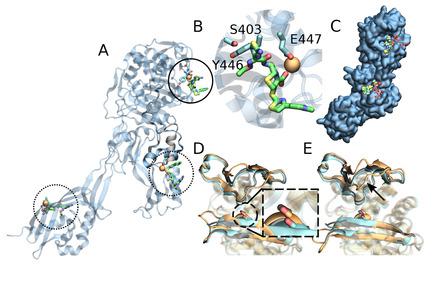
Methicillin‐resistant Staphylococcus aureus (MRSA) tolerates β‐lactam antibiotics by carrying out cell wall synthesis with the transpeptidase Penicillin‐binding protein 2a (PBP2a), which cannot be inhibited by β‐lactams. It has been proposed that PBP2a's active site is protected by two loops to reduce the probability of it binding with β‐lactams. Previous crystallographic studies suggested that this protected active site opens for reaction once a native substrate binds at an allosteric domain of PBP2a. This opening was proposed for the new β‐lactam ceftaroline's mechanism in successfully treating MRSA infections, i. e. by it binding to the allosteric site, thereby opening the active site to inhibition. In this work, we investigate the binding of ceftaroline at this proposed allosteric site using molecular dynamics simulations. Unstable binding was observed using the major force fields CHARMM36 and Amber ff14SB, and free energy calculations were unable to confirm a strong allosteric effect. Our study suggests that the allosteric effect induced by ceftaroline is weak at best.
中文翻译:

青霉素结合蛋白2a(PBP2a)的变构位点抗生素头孢洛林的分子动力学模拟。
耐甲氧西林金黄色葡萄球菌(MRSA)通过与转肽酶青霉素结合蛋白2a(PBP2a)进行细胞壁合成来耐受β-内酰胺抗生素,而β-内酰胺不能抑制这种蛋白。有人提出,PBP2a的活性位点受两个环保护,以降低其与β-内酰胺结合的可能性。先前的晶体学研究表明,一旦天然底物在PBP2a的变构结构域上结合,该受保护的活性位点就会打开反应。建议为新的β内酰胺头孢洛林成功治疗MRSA感染的机制,即 e。通过与变构位点结合,从而使活性位点受到抑制。在这项工作中,我们使用分子动力学模拟研究了头孢洛林在提议的变构位点的结合。使用主力场CHARMM36和Amber ff14SB观察到不稳定的结合,自由能计算无法确认强烈的变构作用。我们的研究表明头孢洛林诱导的变构作用充其量是微弱的。
更新日期:2020-04-16
中文翻译:

青霉素结合蛋白2a(PBP2a)的变构位点抗生素头孢洛林的分子动力学模拟。
耐甲氧西林金黄色葡萄球菌(MRSA)通过与转肽酶青霉素结合蛋白2a(PBP2a)进行细胞壁合成来耐受β-内酰胺抗生素,而β-内酰胺不能抑制这种蛋白。有人提出,PBP2a的活性位点受两个环保护,以降低其与β-内酰胺结合的可能性。先前的晶体学研究表明,一旦天然底物在PBP2a的变构结构域上结合,该受保护的活性位点就会打开反应。建议为新的β内酰胺头孢洛林成功治疗MRSA感染的机制,即 e。通过与变构位点结合,从而使活性位点受到抑制。在这项工作中,我们使用分子动力学模拟研究了头孢洛林在提议的变构位点的结合。使用主力场CHARMM36和Amber ff14SB观察到不稳定的结合,自由能计算无法确认强烈的变构作用。我们的研究表明头孢洛林诱导的变构作用充其量是微弱的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号