当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The mRNA‐Binding Protein HuR Is a Kinetically‐Privileged Electrophile Sensor
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2020-04-29 , DOI: 10.1002/hlca.202000041 Jesse R Poganik 1, 2 , Alexandra K Van Hall-Beauvais 1 , Marcus J C Long 2 , Michael T Disare 2 , Yi Zhao 1 , Yimon Aye 1
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2020-04-29 , DOI: 10.1002/hlca.202000041 Jesse R Poganik 1, 2 , Alexandra K Van Hall-Beauvais 1 , Marcus J C Long 2 , Michael T Disare 2 , Yi Zhao 1 , Yimon Aye 1
Affiliation
|
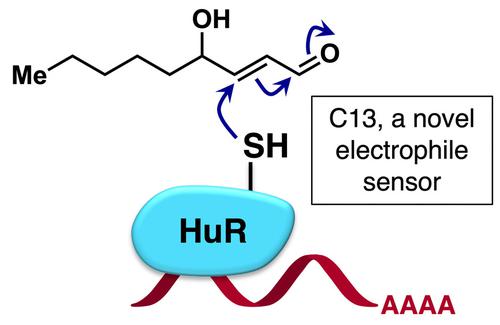
The key mRNA‐binding proteins HuR and AUF1 are reported stress sensors in mammals. Intrigued by recent reports of sensitivity of these proteins to the electrophilic lipid prostaglandin A2 and other redox signals, we here examined their sensing abilities to a prototypical redox‐linked lipid‐derived electrophile, 4‐hydroxynonenal (HNE). Leveraging our T‐REX electrophile delivery platform, we found that only HuR, and not AUF1, is a kinetically‐privileged sensor of HNE in HEK293T cells, and sensing functions through a specific cysteine, C13. Cells depleted of HuR, upon treatment with HNE, manifest unique alterations in cell viability and Nrf2‐transcription‐factor‐driven antioxidant response (AR), which our recent work shows is regulated by HuR at the Nrf2‐mRNA level. Mutagenesis studies showed that C13‐specific sensing alone is not sufficient to explain HuR‐dependent stress responsivities, further highlighting a complex context‐dependent layer of Nrf2/AR regulation through HuR.
中文翻译:

mRNA 结合蛋白 HuR 是一种动力学特权的亲电子传感器
据报道,关键的 mRNA 结合蛋白 HuR 和 AUF1 是哺乳动物的压力传感器。最近关于这些蛋白质对亲电脂质前列腺素 A2 和其他氧化还原信号的敏感性的报道引起了我们的兴趣,我们在这里检查了它们对原型氧化还原连接的脂质衍生亲电试剂 4-羟基壬烯醛 (HNE) 的感知能力。利用我们的 T-REX 亲电传递平台,我们发现只有 HuR 而不是 AUF1,是 HEK293T 细胞中 HNE 的动力学特权传感器,并通过特定的半胱氨酸 C13 进行传感功能。在用 HNE 处理后,缺乏 HuR 的细胞表现出细胞活力和 Nrf2 转录因子驱动的抗氧化反应 (AR) 的独特变化,我们最近的工作表明,HuR 在 Nrf2 mRNA 水平上调节这些变化。
更新日期:2020-04-29
中文翻译:

mRNA 结合蛋白 HuR 是一种动力学特权的亲电子传感器
据报道,关键的 mRNA 结合蛋白 HuR 和 AUF1 是哺乳动物的压力传感器。最近关于这些蛋白质对亲电脂质前列腺素 A2 和其他氧化还原信号的敏感性的报道引起了我们的兴趣,我们在这里检查了它们对原型氧化还原连接的脂质衍生亲电试剂 4-羟基壬烯醛 (HNE) 的感知能力。利用我们的 T-REX 亲电传递平台,我们发现只有 HuR 而不是 AUF1,是 HEK293T 细胞中 HNE 的动力学特权传感器,并通过特定的半胱氨酸 C13 进行传感功能。在用 HNE 处理后,缺乏 HuR 的细胞表现出细胞活力和 Nrf2 转录因子驱动的抗氧化反应 (AR) 的独特变化,我们最近的工作表明,HuR 在 Nrf2 mRNA 水平上调节这些变化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号