当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Human salivary histatin-1 (Hst1) promotes bone morphogenetic protein 2 (BMP2)-induced osteogenesis and angiogenesis.
FEBS Open Bio ( IF 2.6 ) Pub Date : 2020-06-29 , DOI: 10.1002/2211-5463.12906 Ping Sun 1, 2 , Andi Shi 3, 4, 5 , Chenxi Shen 4 , Yi Liu 5 , Gang Wu 3, 6 , Jianying Feng 7
FEBS Open Bio ( IF 2.6 ) Pub Date : 2020-06-29 , DOI: 10.1002/2211-5463.12906 Ping Sun 1, 2 , Andi Shi 3, 4, 5 , Chenxi Shen 4 , Yi Liu 5 , Gang Wu 3, 6 , Jianying Feng 7
Affiliation
|
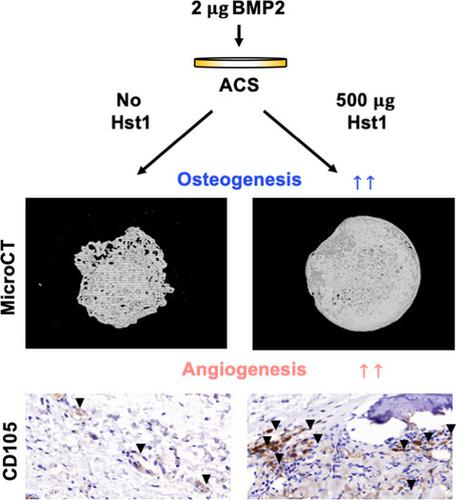
Large‐volume bone defects can result from congenital malformation, trauma, infection, inflammation and cancer. At present, it remains challenging to treat these bone defects with clinically available interventions. Allografts, xenografts and most synthetic materials have no intrinsic osteoinductivity, and so an alternative approach is to functionalize the biomaterial with osteoinductive agents, such as bone morphogenetic protein 2 (BMP2). Because it has been previously demonstrated that human salivary histatin‐1 (Hst1) promotes endothelial cell adhesion, migration and angiogenesis, we examine here whether Hst1 can promote BMP2‐induced bone regeneration. Rats were given subcutaneous implants of absorbable collagen sponge membranes seeded with 0, 50, 200 or 500 μg Hst1 per sample and 0 or 2 μg BMP2 per sample. At 18 days postsurgery, rats were sacrificed, and implanted regional tissue was removed for micro computed tomography (microCT) analyses of new bone (bone volume, trabecular number and trabecular separation). Four samples per group were decalcified and subjected to immunohistochemical staining to analyze osteogenic and angiogenic markers. We observed that Hst1 increased BMP2‐induced new bone formation in a dose‐dependent manner. Co‐administration of 500 μg Hst1 and BMP2 resulted in the highest observed bone volume and trabecular number, the lowest trabecular separation and the highest expression of osteogenic markers and angiogenic markers. Our results suggest that coadministration of Hst1 may enhance BMP2‐induced osteogenesis and angiogenesis, and thus may have potential for development into a treatment for large‐volume bone defects.
中文翻译:

人类唾液组织蛋白 1 (Hst1) 促进骨形态发生蛋白 2 (BMP2) 诱导的成骨和血管生成。
先天性畸形、外伤、感染、炎症和癌症可导致大量骨缺损。目前,通过临床可用的干预措施来治疗这些骨缺损仍然具有挑战性。同种异体移植物、异种移植物和大多数合成材料没有内在的骨诱导性,因此另一种方法是使用骨诱导剂对生物材料进行功能化,例如骨形态发生蛋白 2 (BMP2)。因为之前已经证明人唾液组蛋白-1 (Hst1) 促进内皮细胞粘附、迁移和血管生成,我们在这里检查 Hst1 是否可以促进 BMP2 诱导的骨再生。给大鼠皮下植入可吸收胶原海绵膜,每个样品接种 0、50、200 或 500 μg Hst1,每个样品接种 0 或 2 μg BMP2。术后18天,处死大鼠,取出植入的区域组织用于新骨的显微计算机断层扫描 (microCT) 分析(骨体积、小梁数量和小梁分离)。每组四个样本进行脱钙并进行免疫组织化学染色以分析成骨和血管生成标志物。我们观察到 Hst1 以剂量依赖性方式增加 BMP2 诱导的新骨形成。500 μg Hst1 和 BMP2 的共同给药导致观察到的骨体积和小梁数量最高,小梁分离最低,成骨标志物和血管生成标志物的表达最高。我们的结果表明,Hst1 的共同给药可能会增强 BMP2 诱导的成骨和血管生成,因此可能有潜力发展成为治疗大体积骨缺损的方法。并移除植入的区域组织以对新骨(骨量、小梁数量和小梁分离)进行微计算机断层扫描 (microCT) 分析。每组四个样品进行脱钙并进行免疫组织化学染色以分析成骨和血管生成标志物。我们观察到 Hst1 以剂量依赖性方式增加 BMP2 诱导的新骨形成。500 μg Hst1 和 BMP2 的共同给药导致观察到的骨体积和小梁数量最高,小梁分离最低,成骨标志物和血管生成标志物的表达最高。我们的结果表明,Hst1 的共同给药可能会增强 BMP2 诱导的成骨和血管生成,因此可能有潜力发展成为治疗大体积骨缺损的方法。并移除植入的区域组织以对新骨(骨量、小梁数量和小梁分离)进行微计算机断层扫描 (microCT) 分析。每组四个样品进行脱钙并进行免疫组织化学染色以分析成骨和血管生成标志物。我们观察到 Hst1 以剂量依赖性方式增加 BMP2 诱导的新骨形成。500 μg Hst1 和 BMP2 的共同给药导致观察到的骨体积和小梁数量最高,小梁分离最低,成骨标志物和血管生成标志物的表达最高。我们的结果表明,Hst1 的共同给药可能会增强 BMP2 诱导的成骨和血管生成,因此可能有潜力发展成为治疗大体积骨缺损的方法。
更新日期:2020-06-29
中文翻译:

人类唾液组织蛋白 1 (Hst1) 促进骨形态发生蛋白 2 (BMP2) 诱导的成骨和血管生成。
先天性畸形、外伤、感染、炎症和癌症可导致大量骨缺损。目前,通过临床可用的干预措施来治疗这些骨缺损仍然具有挑战性。同种异体移植物、异种移植物和大多数合成材料没有内在的骨诱导性,因此另一种方法是使用骨诱导剂对生物材料进行功能化,例如骨形态发生蛋白 2 (BMP2)。因为之前已经证明人唾液组蛋白-1 (Hst1) 促进内皮细胞粘附、迁移和血管生成,我们在这里检查 Hst1 是否可以促进 BMP2 诱导的骨再生。给大鼠皮下植入可吸收胶原海绵膜,每个样品接种 0、50、200 或 500 μg Hst1,每个样品接种 0 或 2 μg BMP2。术后18天,处死大鼠,取出植入的区域组织用于新骨的显微计算机断层扫描 (microCT) 分析(骨体积、小梁数量和小梁分离)。每组四个样本进行脱钙并进行免疫组织化学染色以分析成骨和血管生成标志物。我们观察到 Hst1 以剂量依赖性方式增加 BMP2 诱导的新骨形成。500 μg Hst1 和 BMP2 的共同给药导致观察到的骨体积和小梁数量最高,小梁分离最低,成骨标志物和血管生成标志物的表达最高。我们的结果表明,Hst1 的共同给药可能会增强 BMP2 诱导的成骨和血管生成,因此可能有潜力发展成为治疗大体积骨缺损的方法。并移除植入的区域组织以对新骨(骨量、小梁数量和小梁分离)进行微计算机断层扫描 (microCT) 分析。每组四个样品进行脱钙并进行免疫组织化学染色以分析成骨和血管生成标志物。我们观察到 Hst1 以剂量依赖性方式增加 BMP2 诱导的新骨形成。500 μg Hst1 和 BMP2 的共同给药导致观察到的骨体积和小梁数量最高,小梁分离最低,成骨标志物和血管生成标志物的表达最高。我们的结果表明,Hst1 的共同给药可能会增强 BMP2 诱导的成骨和血管生成,因此可能有潜力发展成为治疗大体积骨缺损的方法。并移除植入的区域组织以对新骨(骨量、小梁数量和小梁分离)进行微计算机断层扫描 (microCT) 分析。每组四个样品进行脱钙并进行免疫组织化学染色以分析成骨和血管生成标志物。我们观察到 Hst1 以剂量依赖性方式增加 BMP2 诱导的新骨形成。500 μg Hst1 和 BMP2 的共同给药导致观察到的骨体积和小梁数量最高,小梁分离最低,成骨标志物和血管生成标志物的表达最高。我们的结果表明,Hst1 的共同给药可能会增强 BMP2 诱导的成骨和血管生成,因此可能有潜力发展成为治疗大体积骨缺损的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号