Cell Metabolism ( IF 29.0 ) Pub Date : 2020-06-02 , DOI: 10.1016/j.cmet.2020.05.004 Jing Zhang 1 , Jonathan Muri 2 , Gillian Fitzgerald 1 , Tatiane Gorski 1 , Roberto Gianni-Barrera 3 , Evi Masschelein 1 , Gommaar D'Hulst 1 , Paola Gilardoni 1 , Guillermo Turiel 1 , Zheng Fan 1 , TongTong Wang 4 , Mélanie Planque 5 , Peter Carmeliet 6 , Luc Pellerin 7 , Christian Wolfrum 4 , Sarah-Maria Fendt 5 , Andrea Banfi 8 , Christian Stockmann 9 , Inés Soro-Arnáiz 1 , Manfred Kopf 2 , Katrien De Bock 1
|
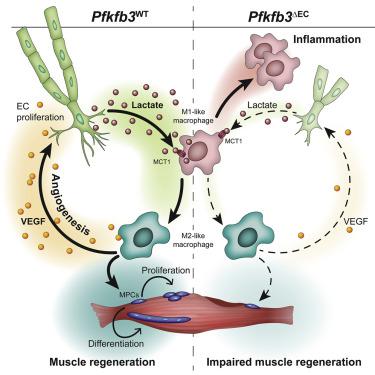
Endothelial cell (EC)-derived signals contribute to organ regeneration, but angiocrine metabolic communication is not described. We found that EC-specific loss of the glycolytic regulator pfkfb3 reduced ischemic hindlimb revascularization and impaired muscle regeneration. This was caused by the reduced ability of macrophages to adopt a proangiogenic and proregenerative M2-like phenotype. Mechanistically, loss of pfkfb3 reduced lactate secretion by ECs and lowered lactate levels in the ischemic muscle. Addition of lactate to pfkfb3-deficient ECs restored M2-like polarization in an MCT1-dependent fashion. Lactate shuttling by ECs enabled macrophages to promote proliferation and fusion of muscle progenitors. Moreover, VEGF production by lactate-polarized macrophages was increased, resulting in a positive feedback loop that further stimulated angiogenesis. Finally, increasing lactate levels during ischemia rescued macrophage polarization and improved muscle reperfusion and regeneration, whereas macrophage-specific mct1 deletion prevented M2-like polarization. In summary, ECs exploit glycolysis for angiocrine lactate shuttling to steer muscle regeneration from ischemia.
中文翻译:

内皮乳酸通过诱导 M2 样巨噬细胞极化来控制缺血引起的肌肉再生。
内皮细胞 (EC) 衍生的信号有助于器官再生,但没有描述血管分泌代谢通讯。我们发现糖酵解调节剂pfkfb3 的EC 特异性丢失减少了缺血性后肢血运重建和肌肉再生受损。这是由于巨噬细胞采用促血管生成和促再生 M2 样表型的能力降低所致。从机制上讲,pfkfb3 的缺失减少了 ECs 的乳酸分泌并降低了缺血肌肉中的乳酸水平。将乳酸添加到pfkfb3- 缺陷的 ECs 以 MCT1 依赖的方式恢复了 M2 样极化。ECs 的乳酸穿梭使巨噬细胞能够促进肌肉祖细胞的增殖和融合。此外,乳酸极化巨噬细胞产生的 VEGF 增加,导致进一步刺激血管生成的正反馈回路。最后,在缺血期间增加乳酸水平挽救了巨噬细胞极化并改善了肌肉再灌注和再生,而巨噬细胞特异性mct1缺失阻止了 M2 样极化。总之,ECs 利用糖酵解进行血管分泌乳酸穿梭来引导肌肉从缺血中再生。


























 京公网安备 11010802027423号
京公网安备 11010802027423号