Cell Metabolism ( IF 29.0 ) Pub Date : 2020-06-02 , DOI: 10.1016/j.cmet.2020.05.008 Rehana Qureshi 1 , Manuel Picon-Ruiz 2 , Iskander Aurrekoetxea-Rodriguez 3 , Vanessa Nunes de Paiva 1 , Massimo D'Amico 1 , Hyunho Yoon 1 , Ramya Radhakrishnan 1 , Cynthia Morata-Tarifa 1 , Tan Ince 4 , Marc E Lippman 5 , Seth R Thaller 6 , Steven E Rodgers 7 , Susan Kesmodel 7 , Maria Del Mar Vivanco 8 , Joyce M Slingerland 9
|
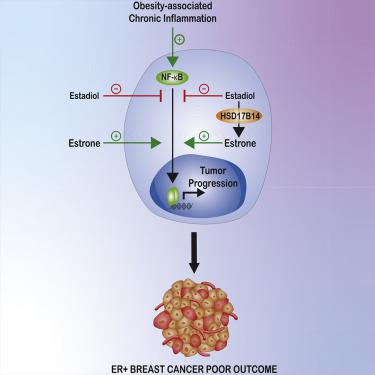
Many inflammation-associated diseases, including cancers, increase in women after menopause and with obesity. In contrast to anti-inflammatory actions of 17β-estradiol, we find estrone, which dominates after menopause, is pro-inflammatory. In human mammary adipocytes, cytokine expression increases with obesity, menopause, and cancer. Adipocyte:cancer cell interaction stimulates estrone- and NFκB-dependent pro-inflammatory cytokine upregulation. Estrone- and 17β-estradiol-driven transcriptomes differ. Estrone:ERα stimulates NFκB-mediated cytokine gene induction; 17β-estradiol opposes this. In obese mice, estrone increases and 17β-estradiol relieves inflammation. Estrone drives more rapid ER+ breast cancer growth in vivo. HSD17B14, which converts 17β-estradiol to estrone, associates with poor ER+ breast cancer outcome. Estrone and HSD17B14 upregulate inflammation, ALDH1 activity, and tumorspheres, while 17β-estradiol and HSD17B14 knockdown oppose these. Finally, a high intratumor estrone:17β-estradiol ratio increases tumor-initiating stem cells and ER+ cancer growth in vivo. These findings help explain why postmenopausal ER+ breast cancer increases with obesity, and offer new strategies for prevention and therapy.
中文翻译:

主要的绝经前和绝经后雌激素在肥胖引起的乳腺炎症和乳腺癌的发展中起着相反的作用。
许多炎症相关疾病,包括癌症,在绝经后和肥胖的女性中增加。与 17β-雌二醇的抗炎作用相反,我们发现绝经后占主导地位的雌酮是促炎的。在人类乳腺脂肪细胞中,细胞因子表达会随着肥胖、更年期和癌症而增加。脂肪细胞:癌细胞相互作用刺激雌酮和 NFκB 依赖性促炎细胞因子上调。雌酮和 17β-雌二醇驱动的转录组不同。雌酮:ERα 刺激 NFκB 介导的细胞因子基因诱导;17β-雌二醇反对这一点。在肥胖小鼠中,雌酮增加,17β-雌二醇减轻炎症。雌酮在体内驱动更快速的 ER+ 乳腺癌生长. HSD17B14 将 17β-雌二醇转化为雌酮,与不良的 ER+ 乳腺癌预后相关。雌酮和 HSD17B14 上调炎症、ALDH1 活性和肿瘤球,而 17β-雌二醇和HSD17B14敲低则相反。最后,较高的瘤内雌酮:17β-雌二醇比率可增加体内肿瘤起始干细胞和 ER+ 癌症的生长。这些发现有助于解释为什么绝经后 ER+ 乳腺癌会随着肥胖而增加,并为预防和治疗提供新的策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号