当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Phase Equilibria of the Quaternary System MgCl2–PbCl2–ZnCl2–H2O at 323 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-06-01 , DOI: 10.1021/acs.jced.0c00200 Yun-Yun Gao 1 , Xiao-Feng He 1 , Shi-Hua Sang 1, 2 , Xiao-Feng Guo 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-06-01 , DOI: 10.1021/acs.jced.0c00200 Yun-Yun Gao 1 , Xiao-Feng He 1 , Shi-Hua Sang 1, 2 , Xiao-Feng Guo 1
Affiliation
|
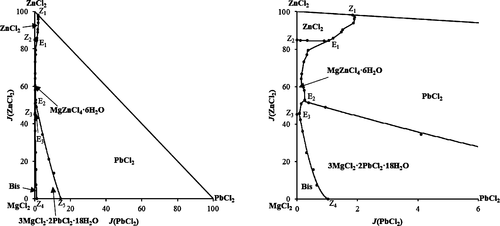
To provide theoretical data for the hydrometallurgical process of lead–zinc ores and the separation process of secondary lead recovery, the quaternary system MgCl2–PbCl2–ZnCl2–H2O at 323 K was investigated using the isothermal dissolution equilibrium method. The isothermal solubility data were obtained, based on which the corresponding dry salt phase diagram and the water content diagram were constructed. The double salts MgZnCl4·6H2O and 3MgCl2·2PbCl2·18H2O were found in the equilibrium solid phases as its ternary subsystems. The dry salt phase diagram contains three invariant points, seven isothermal solubility curves, and five crystallization fields (the crystal salts are PbCl2, 3MgCl2·2PbCl2·18H2O, ZnCl2, MgZnCl4·6H2O, and MgCl2·6H2O(Bis), respectively), and the crystallization region of PbCl2 is the largest. At the same time, the invariant data of this quaternary system and its ternary subsystems at different temperatures were compared and discussed.
中文翻译:

323 K时四元体系MgCl 2 -PbCl 2 -ZnCl 2 -H 2 O的固液平衡
以提供用于铅-锌矿石的湿法冶金工艺理论数据和次级铅回收的分离过程中,四元体系的MgCl 2 -PbCl 2 -ZnCl 2 -H 2 O不连到323 K,采用等温溶解平衡法研究。获得了等温溶解度数据,在此基础上构建了相应的干盐相图和水含量图。复盐MgZnCl 4 ·6H 2 O和3MgCl 2 ·2PbCl 2 ·18H 2O在平衡固相中作为其三元子系统被发现。将干燥的盐的相图包含三个不变点,七条温溶解度曲线,和五个结晶区(晶体盐是PBCL 2,3MgCl 2 ·2PbCl 2 ·18H 2 O,氯化锌2,MgZnCl 4 ·6H 2 O,和氯化镁2 ·6H 2 O(Bis),并且PbCl 2的结晶区域最大。同时,比较并讨论了该四元系统及其三元子系统在不同温度下的不变数据。
更新日期:2020-06-01
中文翻译:

323 K时四元体系MgCl 2 -PbCl 2 -ZnCl 2 -H 2 O的固液平衡
以提供用于铅-锌矿石的湿法冶金工艺理论数据和次级铅回收的分离过程中,四元体系的MgCl 2 -PbCl 2 -ZnCl 2 -H 2 O不连到323 K,采用等温溶解平衡法研究。获得了等温溶解度数据,在此基础上构建了相应的干盐相图和水含量图。复盐MgZnCl 4 ·6H 2 O和3MgCl 2 ·2PbCl 2 ·18H 2O在平衡固相中作为其三元子系统被发现。将干燥的盐的相图包含三个不变点,七条温溶解度曲线,和五个结晶区(晶体盐是PBCL 2,3MgCl 2 ·2PbCl 2 ·18H 2 O,氯化锌2,MgZnCl 4 ·6H 2 O,和氯化镁2 ·6H 2 O(Bis),并且PbCl 2的结晶区域最大。同时,比较并讨论了该四元系统及其三元子系统在不同温度下的不变数据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号