当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Methane Pyrolysis in Molten Alkali Chloride Salts Containing Iron
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-06-01 , DOI: 10.1021/acscatal.0c01262 Dohyung Kang 1 , Clarke Palmer 2 , Davide Mannini 2 , Nazanin Rahimi 2 , Michael J. Gordon 2 , Horia Metiu 3 , Eric W. McFarland 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-06-01 , DOI: 10.1021/acscatal.0c01262 Dohyung Kang 1 , Clarke Palmer 2 , Davide Mannini 2 , Nazanin Rahimi 2 , Michael J. Gordon 2 , Horia Metiu 3 , Eric W. McFarland 2
Affiliation
|
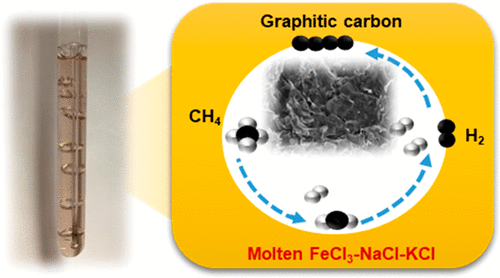
Mixtures of molten iron–sodium-potassium chloride salts are found to be catalytic for methane pyrolysis. In a differential bubble column reactor, the apparent activation energy of the molten salt decreases from 301 kJ/mol for the eutectic NaCl-KCl to 171 kJ/mol for 3 wt % of iron-added as FeCl3. The solid carbon produced in the iron-containing salt mixture has a graphitic structure which is distinct from the more disordered carbon produced in the iron-free eutectic, suggesting a different solid carbon formation pathway. Results from H–D exchange investigations are consistent with a different reaction pathway for methane pyrolysis in the iron-containing NaCl-KCl melt than in the melt without Fe. The activity of the salt mixture was stable for over 50 h, producing molecular hydrogen and separable solid carbon. It is likely that the activity is due to the presence of Fe in molecular ions stabilized in the NaCl-KCl melt that facilitate the C–H bond activation in methane.
中文翻译:

含铁熔融氯碱盐中的甲烷催化热解
发现熔融铁-氯化钠-钾盐的混合物对甲烷热解具有催化作用。在差动鼓泡塔反应器中,熔盐的表观活化能从低共熔NaCl-KCl的301 kJ / mol降低到3 wt%的铁作为FeCl 3添加的171 kJ / mol。。含铁盐混合物中产生的固体碳具有石墨结构,这与无铁共晶中产生的无序碳不同,这表明存在不同的固体碳形成途径。H-D交换研究的结果与含铁的NaCl-KCl熔体中的甲烷热解反应与不含Fe的熔体中甲烷热解反应的反应路径不同是一致的。盐混合物的活性在超过50小时内保持稳定,产生了分子氢和可分离的固体碳。活性可能是由于在NaCl-KCl熔体中稳定的分子离子中存在Fe,从而促进了甲烷中C–H键的活化。
更新日期:2020-07-02
中文翻译:

含铁熔融氯碱盐中的甲烷催化热解
发现熔融铁-氯化钠-钾盐的混合物对甲烷热解具有催化作用。在差动鼓泡塔反应器中,熔盐的表观活化能从低共熔NaCl-KCl的301 kJ / mol降低到3 wt%的铁作为FeCl 3添加的171 kJ / mol。。含铁盐混合物中产生的固体碳具有石墨结构,这与无铁共晶中产生的无序碳不同,这表明存在不同的固体碳形成途径。H-D交换研究的结果与含铁的NaCl-KCl熔体中的甲烷热解反应与不含Fe的熔体中甲烷热解反应的反应路径不同是一致的。盐混合物的活性在超过50小时内保持稳定,产生了分子氢和可分离的固体碳。活性可能是由于在NaCl-KCl熔体中稳定的分子离子中存在Fe,从而促进了甲烷中C–H键的活化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号