当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Binding modes of thioflavin T and Congo red to the fibril structure of amyloid-β(1-42).
Chemical Communications ( IF 4.9 ) Pub Date : 2020-06-01 , DOI: 10.1039/d0cc01161d Benedikt Frieg 1 , Lothar Gremer , Henrike Heise , Dieter Willbold , Holger Gohlke
Chemical Communications ( IF 4.9 ) Pub Date : 2020-06-01 , DOI: 10.1039/d0cc01161d Benedikt Frieg 1 , Lothar Gremer , Henrike Heise , Dieter Willbold , Holger Gohlke
Affiliation
|
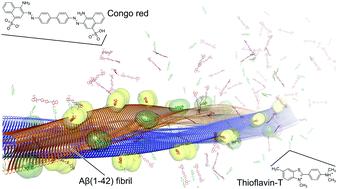
Binding modes for the amyloid-β(1–42) fibril fluorescent dyes thioflavin T and Congo red were predicted by molecular dynamics simulations and binding free energy calculations. Both probes bind on the fibril surface to primarily hydrophobic grooves, with their long axis oriented almost parallel to the fibril axis. The computed binding affinities are in agreement with experimental values. The binding modes also explain observables from previous structural studies and, thus, provide a starting point for the systematic search and design of novel molecules, which may improve in vitro diagnostics for Alzheimer's disease.
中文翻译:

硫黄素T和刚果红与淀粉样蛋白β(1-42)的原纤维结构的结合方式。
通过分子动力学模拟和结合自由能计算,预测了淀粉样β-(1-42)原纤维荧光染料硫黄素T和刚果红的结合模式。两种探针均在原纤维表面上结合至主要的疏水性凹槽,其长轴方向几乎平行于原纤维轴。计算的结合亲和力与实验值一致。结合模式还解释了以前的结构研究中的可观察到的结果,因此为系统搜索和设计新分子提供了起点,这可能会改善阿尔茨海默氏病的体外诊断。
更新日期:2020-07-09
中文翻译:

硫黄素T和刚果红与淀粉样蛋白β(1-42)的原纤维结构的结合方式。
通过分子动力学模拟和结合自由能计算,预测了淀粉样β-(1-42)原纤维荧光染料硫黄素T和刚果红的结合模式。两种探针均在原纤维表面上结合至主要的疏水性凹槽,其长轴方向几乎平行于原纤维轴。计算的结合亲和力与实验值一致。结合模式还解释了以前的结构研究中的可观察到的结果,因此为系统搜索和设计新分子提供了起点,这可能会改善阿尔茨海默氏病的体外诊断。



























 京公网安备 11010802027423号
京公网安备 11010802027423号