当前位置:
X-MOL 学术
›
Microb. Biotechnol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Safety of photosynthetic Synechococcus elongatus for in vivo cyanobacteria-mammalian symbiotic therapeutics.
Microbial Biotechnology ( IF 5.7 ) Pub Date : 2020-05-31 , DOI: 10.1111/1751-7915.13596 Kiah M Williams 1 , Hanjay Wang 1 , Michael J Paulsen 1 , Akshara D Thakore 1 , Mary Rieck 2 , Haley J Lucian 1 , Frederick Grady 1 , Camille E Hironaka 1 , Athena J Chien 1 , Justin M Farry 1 , Hye Sook Shin 1 , Kevin J Jaatinen 1 , Anahita Eskandari 1 , Lyndsay M Stapleton 1, 3 , Amanda N Steele 1, 3 , Jeffrey E Cohen 1 , Y Joseph Woo 1, 3
Microbial Biotechnology ( IF 5.7 ) Pub Date : 2020-05-31 , DOI: 10.1111/1751-7915.13596 Kiah M Williams 1 , Hanjay Wang 1 , Michael J Paulsen 1 , Akshara D Thakore 1 , Mary Rieck 2 , Haley J Lucian 1 , Frederick Grady 1 , Camille E Hironaka 1 , Athena J Chien 1 , Justin M Farry 1 , Hye Sook Shin 1 , Kevin J Jaatinen 1 , Anahita Eskandari 1 , Lyndsay M Stapleton 1, 3 , Amanda N Steele 1, 3 , Jeffrey E Cohen 1 , Y Joseph Woo 1, 3
Affiliation
|
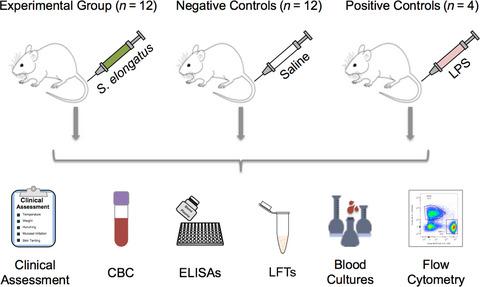
The cyanobacterium Synechococcus elongatus (SE) has been shown to rescue ischaemic heart muscle after myocardial infarction by photosynthetic oxygen production. Here, we investigated SE toxicity and hypothesized that systemic SE exposure does not elicit a significant immune response in rats. Wistar rats intravenously received SE (n = 12), sterile saline (n = 12) or E. coli lipopolysaccharide (LPS, n = 4), and a subset (8 SE, 8 saline) received a repeat injection 4 weeks later. At baseline, 4 h, 24 h, 48 h, 8 days and 4 weeks after injection, clinical assessments, blood cultures, blood counts, lymphocyte phenotypes, liver function tests, proinflammatory cytokines and immunoglobulins were assessed. Across all metrics, SE rats responded comparably to saline controls, displaying no clinically significant immune response. As expected, LPS rats exhibited severe immunological responses. Systemic SE administration does not induce sepsis or toxicity in rats, thereby supporting the safety of cyanobacteria–mammalian symbiotic therapeutics using this organism.
中文翻译:

光合细长聚球藻用于体内蓝藻-哺乳动物共生疗法的安全性。
蓝藻细长聚球藻(SE) 已被证明可以通过光合作用产生氧气来挽救心肌梗塞后缺血的心肌。在这里,我们研究了 SE 毒性,并假设全身性 SE 暴露不会在大鼠中引起显着的免疫反应。Wistar 大鼠静脉注射 SE(n = 12)、无菌盐水(n = 12)或大肠杆菌脂多糖(LPS,n = 4),其中一部分(8 只 SE、8 只盐水)在 4 周后接受重复注射。在基线、注射后4小时、24小时、48小时、8天和4周时,评估临床评估、血培养、血细胞计数、淋巴细胞表型、肝功能测试、促炎细胞因子和免疫球蛋白。在所有指标中,SE 大鼠的反应与盐水对照组相当,没有表现出具有临床意义的免疫反应。正如预期的那样,LPS 大鼠表现出严重的免疫反应。全身性 SE 给药不会诱发大鼠败血症或毒性,从而支持使用该生物体的蓝藻-哺乳动物共生疗法的安全性。
更新日期:2020-05-31
中文翻译:

光合细长聚球藻用于体内蓝藻-哺乳动物共生疗法的安全性。
蓝藻细长聚球藻(SE) 已被证明可以通过光合作用产生氧气来挽救心肌梗塞后缺血的心肌。在这里,我们研究了 SE 毒性,并假设全身性 SE 暴露不会在大鼠中引起显着的免疫反应。Wistar 大鼠静脉注射 SE(n = 12)、无菌盐水(n = 12)或大肠杆菌脂多糖(LPS,n = 4),其中一部分(8 只 SE、8 只盐水)在 4 周后接受重复注射。在基线、注射后4小时、24小时、48小时、8天和4周时,评估临床评估、血培养、血细胞计数、淋巴细胞表型、肝功能测试、促炎细胞因子和免疫球蛋白。在所有指标中,SE 大鼠的反应与盐水对照组相当,没有表现出具有临床意义的免疫反应。正如预期的那样,LPS 大鼠表现出严重的免疫反应。全身性 SE 给药不会诱发大鼠败血症或毒性,从而支持使用该生物体的蓝藻-哺乳动物共生疗法的安全性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号