当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cre/loxP approach-mediated downregulation of Pik3c3 inhibits the hypertrophic growth of renal proximal tubule cells.
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2020-05-31 , DOI: 10.1002/jcp.29811 Ting Liu 1 , Jialing Yuan 1 , Caihong Dai 1 , Jinxian Xu 1 , Shude Li 2 , Benjamin D Humphreys 3 , Daniel T Kleven 4 , Jian-Kang Chen 1
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2020-05-31 , DOI: 10.1002/jcp.29811 Ting Liu 1 , Jialing Yuan 1 , Caihong Dai 1 , Jinxian Xu 1 , Shude Li 2 , Benjamin D Humphreys 3 , Daniel T Kleven 4 , Jian-Kang Chen 1
Affiliation
|
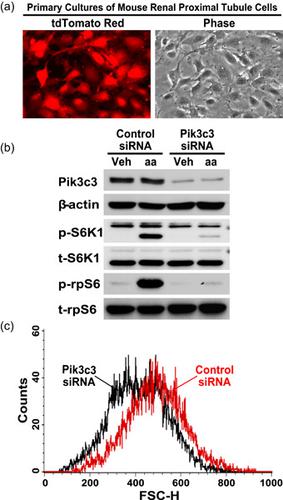
Nephron loss stimulates residual functioning nephrons to undergo compensatory growth. Excessive nephron growth may be a maladaptive response that sets the stage for progressive nephron damage, leading to kidney failure. To date, however, the mechanism of nephron growth remains incompletely understood. Our previous study revealed that class III phosphatidylinositol‐3‐kinase (Pik3c3) is activated in the remaining kidney after unilateral nephrectomy (UNX)‐induced nephron loss, but previous studies failed to generate a Pik3c3 gene knockout animal model. Global Pik3c3 deletion results in embryonic lethality. Given that renal proximal tubule cells make up the bulk of the kidney and undergo the most prominent hypertrophic growth after UNX, in this study we used Cre‐loxP‐based approaches to demonstrate for the first time that tamoxifen‐inducible SLC34a1 promoter‐driven CreERT2 recombinase‐mediated downregulation of Pik3c3 expression in renal proximal tubule cells alone is sufficient to inhibit UNX‐ or amino acid‐induced hypertrophic nephron growth. Furthermore, our mechanistic studies unveiled that the SLC34a1‐CreERT2 recombinase‐mediated Pik3c3 downregulation inhibited UNX‐ or amino acid‐stimulated lysosomal localization and signaling activation of mechanistic target of rapamycin complex 1 (mTORC1) in the renal proximal tubules. Moreover, our additional cell culture experiments using RNAi confirmed that knocking down Pik3c3 expression inhibited amino acid‐stimulated mTORC1 signaling and blunted cellular growth in primary cultures of renal proximal tubule cells. Together, both our in vivo and in vitro experimental results indicate that Pik3c3 is a major mechanistic mediator responsible for sensing amino acid availability and initiating hypertrophic growth of renal proximal tubule cells by activation of the mTORC1–S6K1–rpS6 signaling pathway.
中文翻译:

Cre/loxP 方法介导的 Pik3c3 下调抑制肾近端小管细胞的肥大生长。
肾单位损失刺激残余功能肾单位进行代偿性生长。肾单位过度生长可能是一种适应不良反应,为进行性肾单位损伤奠定了基础,导致肾功能衰竭。然而,迄今为止,对肾单位生长的机制仍未完全了解。我们之前的研究表明,单侧肾切除术 (UNX) 诱导的肾单位丢失后,剩余肾脏中 III 类磷脂酰肌醇 3 激酶 (Pik3c3) 被激活,但之前的研究未能生成Pik3c3基因敲除动物模型。全球Pik3c3缺失导致胚胎致死。鉴于肾近端小管细胞构成肾脏的大部分并在 UNX 后经历最显着的肥大生长,在本研究中,我们使用基于 Cre-loxP 的方法首次证明他莫昔芬诱导的SLC34a1启动子驱动的CreER T2仅在肾近端小管细胞中重组酶介导的 Pik3c3 表达下调就足以抑制 UNX 或氨基酸诱导的肥大性肾单位生长。此外,我们的机械研究表明SLC34a1 ‐ CreER T2重组酶介导的 Pik3c3 下调抑制了 UNX 或氨基酸刺激的溶酶体定位和肾近端小管中雷帕霉素复合物 1 (mTORC1) 机制靶点的信号激活。此外,我们使用 RNAi 进行的额外细胞培养实验证实,在肾近端小管细胞的原代培养物中,敲低 Pik3c3 表达可抑制氨基酸刺激的 mTORC1 信号传导并减弱细胞生长。总之,我们的体内和体外实验结果表明,Pik3c3 是负责感知氨基酸可用性和通过激活 mTORC1-S6K1-rpS6 信号通路启动肾近端小管细胞肥大生长的主要机制介质。
更新日期:2020-05-31
中文翻译:

Cre/loxP 方法介导的 Pik3c3 下调抑制肾近端小管细胞的肥大生长。
肾单位损失刺激残余功能肾单位进行代偿性生长。肾单位过度生长可能是一种适应不良反应,为进行性肾单位损伤奠定了基础,导致肾功能衰竭。然而,迄今为止,对肾单位生长的机制仍未完全了解。我们之前的研究表明,单侧肾切除术 (UNX) 诱导的肾单位丢失后,剩余肾脏中 III 类磷脂酰肌醇 3 激酶 (Pik3c3) 被激活,但之前的研究未能生成Pik3c3基因敲除动物模型。全球Pik3c3缺失导致胚胎致死。鉴于肾近端小管细胞构成肾脏的大部分并在 UNX 后经历最显着的肥大生长,在本研究中,我们使用基于 Cre-loxP 的方法首次证明他莫昔芬诱导的SLC34a1启动子驱动的CreER T2仅在肾近端小管细胞中重组酶介导的 Pik3c3 表达下调就足以抑制 UNX 或氨基酸诱导的肥大性肾单位生长。此外,我们的机械研究表明SLC34a1 ‐ CreER T2重组酶介导的 Pik3c3 下调抑制了 UNX 或氨基酸刺激的溶酶体定位和肾近端小管中雷帕霉素复合物 1 (mTORC1) 机制靶点的信号激活。此外,我们使用 RNAi 进行的额外细胞培养实验证实,在肾近端小管细胞的原代培养物中,敲低 Pik3c3 表达可抑制氨基酸刺激的 mTORC1 信号传导并减弱细胞生长。总之,我们的体内和体外实验结果表明,Pik3c3 是负责感知氨基酸可用性和通过激活 mTORC1-S6K1-rpS6 信号通路启动肾近端小管细胞肥大生长的主要机制介质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号