当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sustainable short-chain olefin production through simultaneous dehydration of mixtures of 1-butanol and ethanol over HZSM-5 and γ-Al2O3
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.jiec.2020.05.022 Arno de Reviere , Dieter Gunst , Maarten Sabbe , An Verberckmoes
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.jiec.2020.05.022 Arno de Reviere , Dieter Gunst , Maarten Sabbe , An Verberckmoes
|
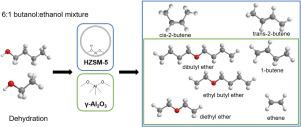
Abstract Dehydration reactions of alcohols are of prime importance in biomass conversions. Although bio-alcohols are generally obtained as mixtures, the dehydration of alcohol mixtures is barely reported. In this work the ability of HZSM-5 and γ-Al2O3 to dehydrate a 1-butanol/ethanol mixture in a 6/1 mass-ratio was studied, corresponding to the butanol/ethanol ratio in typical ABE mixtures, at temperatures from 513 to 613 K. For HZSM-5 the difference in reactivity of the alcohols is too high to fully convert both alcohols to their respective alkenes without catalyzing secondary reactions. For dehydration over γ-Al2O3, the reactivity of ethanol and 1-butanol is more similar, which can be attributed to the lower acid strength and larger pore size, causing lower fractional coverages and diminished competition for adsorption sites, allowing both alcohols to react more simultaneously. Above conversions of 0.6, the difference in reactivity of both alcohols increases due to a shift in dominant reaction pathways towards ether decomposition and the intramolecular dehydration on γ-Al2O3. Approaching full conversion, the selectivity towards olefins is high (>0.95) for both HZSM-5 and γ-Al2O3. Since no secondary reactions occur when using γ-Al2O3, it is deemed the best of both catalysts to dehydrate mixtures of alcohols simultaneously to their corresponding alkenes.
中文翻译:

通过在 HZSM-5 和 γ-Al2O3 上同时脱水 1-丁醇和乙醇的混合物,可持续生产短链烯烃
摘要 醇的脱水反应在生物质转化中至关重要。虽然生物醇通常以混合物的形式获得,但几乎没有报道醇混合物的脱水。在这项工作中,研究了 HZSM-5 和 γ-Al2O3 对 6/1 质量比的 1-丁醇/乙醇混合物进行脱水的能力,对应于典型 ABE 混合物中的丁醇/乙醇比,温度为 513 至613 K. 对于 HZSM-5,醇的反应性差异太高,无法在不催化二次反应的情况下将两种醇完全转化为各自的烯烃。对于 γ-Al2O3 脱水,乙醇和 1-丁醇的反应性更相似,这可归因于较低的酸强度和较大的孔径,导致较低的覆盖率和对吸附位点的竞争减少,允许两种醇同时反应。高于 0.6 的转化率,由于主要反应途径转向醚分解和 γ-Al2O3 的分子内脱水,两种醇的反应性差异增加。接近完全转化时,HZSM-5 和 γ-Al2O3 对烯烃的选择性很高 (>0.95)。由于使用 γ-Al2O3 时不会发生二次反应,因此两种催化剂中最好的一种是同时将醇的混合物脱水为其相应的烯烃。95) 对于 HZSM-5 和 γ-Al2O3。由于使用 γ-Al2O3 时不会发生二次反应,因此两种催化剂中最好的一种是同时将醇的混合物脱水为其相应的烯烃。95) 对于 HZSM-5 和 γ-Al2O3。由于使用 γ-Al2O3 时不会发生二次反应,因此两种催化剂中最好的一种是同时将醇的混合物脱水为其相应的烯烃。
更新日期:2020-09-01
中文翻译:

通过在 HZSM-5 和 γ-Al2O3 上同时脱水 1-丁醇和乙醇的混合物,可持续生产短链烯烃
摘要 醇的脱水反应在生物质转化中至关重要。虽然生物醇通常以混合物的形式获得,但几乎没有报道醇混合物的脱水。在这项工作中,研究了 HZSM-5 和 γ-Al2O3 对 6/1 质量比的 1-丁醇/乙醇混合物进行脱水的能力,对应于典型 ABE 混合物中的丁醇/乙醇比,温度为 513 至613 K. 对于 HZSM-5,醇的反应性差异太高,无法在不催化二次反应的情况下将两种醇完全转化为各自的烯烃。对于 γ-Al2O3 脱水,乙醇和 1-丁醇的反应性更相似,这可归因于较低的酸强度和较大的孔径,导致较低的覆盖率和对吸附位点的竞争减少,允许两种醇同时反应。高于 0.6 的转化率,由于主要反应途径转向醚分解和 γ-Al2O3 的分子内脱水,两种醇的反应性差异增加。接近完全转化时,HZSM-5 和 γ-Al2O3 对烯烃的选择性很高 (>0.95)。由于使用 γ-Al2O3 时不会发生二次反应,因此两种催化剂中最好的一种是同时将醇的混合物脱水为其相应的烯烃。95) 对于 HZSM-5 和 γ-Al2O3。由于使用 γ-Al2O3 时不会发生二次反应,因此两种催化剂中最好的一种是同时将醇的混合物脱水为其相应的烯烃。95) 对于 HZSM-5 和 γ-Al2O3。由于使用 γ-Al2O3 时不会发生二次反应,因此两种催化剂中最好的一种是同时将醇的混合物脱水为其相应的烯烃。



























 京公网安备 11010802027423号
京公网安备 11010802027423号