Current Computer-Aided Drug Design ( IF 1.7 ) Pub Date : 2021-05-31 , DOI: 10.2174/1573409916666200527133126 Reihaneh Heidarian 1 , Mansoureh Zahedi-Tabrizi 1
|
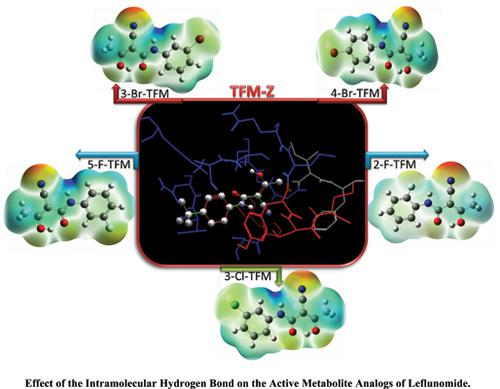
Background: Leflunomide (LFM) and its active metabolite, teriflunomide (TFM), have drawn a lot of attention for their anticancer activities, treatment of rheumatoid arthritis and malaria due to their capability to inhibit dihydroorotate dehydrogenase (DHODH) and Plasmodium falciparum dihydroorotate dehydrogenase (PfDHODH) enzyme. In this investigation, the strength of intramolecular hydrogen bond (IHB) in five analogs of TFM (ATFM) was analyzed employing density functional theory (DFT) using B3LYP/6-311++G (d, p) level and molecular orbital analysis in the gas phase and water solution. A detailed electronic structure study was performed using the quantum theory of atoms in molecules (QTAIM) and the hydrogen bond energies (EHB) of stable conformer obtained in the range of 76-97 kJ/mol, as a medium hydrogen bond. The effect of substitution on the IHB nature was studied by natural bond orbital analysis (NBO). 1H NMR calculations showed an upward trend in the proton chemical shift of the enolic proton in the chelated ring (14.5 to 15.7ppm) by increasing the IHB strength. All the calculations confirmed the strongest IHB in 5-F-ATFM and the weakest IHB in 2-FATFM. Molecular orbital analysis, including the HOMO-LUMO gap and chemical hardness, was performed to compare the reactivity of inhibitors. Finally, molecular docking analysis was carried out to identify the potency of inhibition of these compounds against PfDHODH enzyme.
TFM acts as an inhibitor of dihydroorotate dehydrogenase (DHODH) and Plasmodium falciparum dihydroorotate dehydrogenase (PfDHODH) enzyme. Leflunomide and its active metabolite teriflunomide have been identified as drugs for treatment of some diseases, such as multiple sclerosis (MS), rheumatoid arthritis (RA), malaria, and cancer. Hydrogen bonds play a key role in the interaction between drugs and enzymes.
Objectives: The aim of the present work is to investigate the effect of the strength of intramolecular hydrogen bonds (IHBs) in the active metabolite analogs of leflunomide or analogs of teriflunomide (ATFMs) and study the interaction of these inhibitors against the PfDHODH enzyme using quantum mechanical methods.
Methods: At first, intramolecular hydrogen bonds in five ATFMs were evaluated by the DFT method, quantum theory of atoms in molecules (QTAIM), nuclear magnetic resonance (NMR), natural bond orbital (NBO), and molecular orbital (MO) analyses. Then, the interaction of these inhibitors against the PfDHODH enzyme were compared using molecular docking study.
Results: All the computed results confirm the following trend in the intramolecular hydrogen bond strength in five mono-halo-substituted 2-cyano-3-hydroxy-N-phenylbut-2-enamide (ATFM): 5-FATFM> 4-Br-ATFM ≈ 3-Br-ATFM>3-Cl-ATFM>TFM-Z>2-F-ATFM which is in agreement with QTAIM, NMR, and NBO results. Docking results show that 5-F-ATFM (EHB=97kJ/mol) has the minimum MolDock score due to its considerable IHB strength.
Conclusion: For strong IHBs (EHB>100kJ/mol), C=O and O–H group are involved in the intramolecular interactions and do not contribute to the external interactions. Also, the docking study revealed maximum binding energy between TFM-Z and PfDHODH enzyme.
中文翻译:

分子内氢键对来氟米特活性代谢物类似物阻断恶性疟原虫二氢乳清酸脱氢酶的影响:QTAIM、NBO 和对接研究
背景:来氟米特 (LFM) 及其活性代谢物特立氟胺 (TFM) 因其能够抑制二氢乳清酸脱氢酶 (DHODH) 和恶性疟原虫二氢乳清酸脱氢酶的能力,因其抗癌活性、治疗类风湿性关节炎和疟疾而备受关注。 PfDHODH) 酶。在这项研究中,使用密度泛函理论 (DFT) 使用 B3LYP/6-311++G (d, p) 水平和分子轨道分析分析了 TFM (ATFM) 的五种类似物的分子内氢键 (IHB) 强度。气相和水溶液。使用分子中原子的量子理论 (QTAIM) 和在 76-97 kJ/mol 范围内获得的稳定构象异构体的氢键能 (EHB) 作为中等氢键进行了详细的电子结构研究。1 H NMR 计算表明,通过增加 IHB 强度,螯合环中烯醇质子的质子化学位移呈上升趋势(14.5 至 15.7ppm)。所有的计算都证实了 5-F-ATFM 中 IHB 最强,而 2-FATFM 中 IHB 最弱。进行分子轨道分析,包括 HOMO-LUMO 间隙和化学硬度,以比较抑制剂的反应性。最后,进行分子对接分析以鉴定这些化合物对 PfDHODH 酶的抑制效力。
TFM 作为二氢乳清酸脱氢酶 (DHODH) 和恶性疟原虫二氢乳清酸脱氢酶 (PfDHODH) 酶的抑制剂。来氟米特及其活性代谢物特立氟胺已被确定为治疗某些疾病的药物,例如多发性硬化症 (MS)、类风湿性关节炎 (RA)、疟疾和癌症。氢键在药物和酶的相互作用中起着关键作用。
目的:本工作的目的是研究来氟米特活性代谢物类似物或特立氟胺类似物 (ATFM) 中分子内氢键 (IHB) 强度的影响,并使用量子技术研究这些抑制剂对 PfDHODH 酶的相互作用。机械方法。
方法:首先,通过 DFT 方法、分子中原子的量子理论 (QTAIM)、核磁共振 (NMR)、自然键轨道 (NBO) 和分子轨道 (MO) 分析评估了五种 ATFM 中的分子内氢键。然后,使用分子对接研究比较了这些抑制剂对 PfDHODH 酶的相互作用。
结果:所有计算结果证实了五种单卤代2-氰基-3-羟基-N-苯基丁-2-烯酰胺(ATFM)分子内氢键强度的趋势:5-FATFM>4-Br- ATFM ≈ 3-Br-ATFM>3-Cl-ATFM>TFM-Z>2-F-ATFM,这与 QTAIM、NMR 和 NBO 结果一致。对接结果表明,5-F-ATFM(E HB =97kJ/mol)由于其相当大的IHB强度而具有最低的MolDock得分。
结论:对于强 IHBs(E HB >100kJ/mol),C=O 和 O-H 基团参与分子内相互作用,对外部相互作用没有贡献。此外,对接研究揭示了 TFM-Z 和 PfDHODH 酶之间的最大结合能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号