The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-05-30 , DOI: 10.1016/j.jct.2020.106179 Mihalj Poša , Ana Pilipović
|
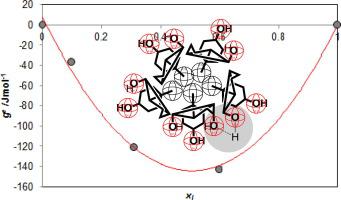
Micellisation of a binary mixture of sodium deoxycholate (SD) and sodium cholate (SC) is examined for the aqueous solution without additives (referent system for self- association process) and for the aqueous solution containing methanol, NaCl and methanol + NaCl in the temperature range T = (278.15–318.15) K. Critical micelle concentrations of mono-component surfactants and binary mixtures of surfactants at different molar ratios of mixing were determined experimentally. These values were used to calculate the mole fractions of building units in the binary mixed micelle, the interaction parameter, the molar excess Gibbs energy and the molar Gibbs energy of micellisation. Effects of additives in aqueous surfactants solution on the micellisation of the binary mixture can be observed in two ways, by considering the excess molar Gibbs energy () or by analyzing the molar Gibbs energy of micellisation (). The impact of methanol in aqueous solution is expressed differently over and while the effect of Na+ ion is similar for both thermodynamic functions: and . In this paper is performed a detailed analysis of the molar Gibbs energies of micellisation: and and their different loading with absolute errors.
中文翻译:

在温度区间T =(278.15–318.15)中,表面活性剂水溶液中的添加剂(甲醇和NaCl)对脱氧胆酸钠和胆酸钠二元混合物胶束化的影响:胶束形成的摩尔过量吉布斯能和摩尔吉布斯能
检查脱氧胆酸钠(SD)和胆酸钠(SC)的二元混合物的胶束化过程,以检测无添加剂的水溶液(自缔合过程的参考系统)以及温度下含甲醇,NaCl和甲醇+ NaCl的水溶液范围T =(278.15–318.15)K.通过实验确定不同混合摩尔比下单组分表面活性剂和表面活性剂的二元混合物的临界胶束浓度。这些值用于计算二元混合胶束中建筑单元的摩尔分数,相互作用参数,胶束化的摩尔过量吉布斯能量和摩尔吉布斯能量。通过考虑过量的摩尔吉布斯能量,可以通过两种方法观察表面活性剂水溶液中添加剂对二元混合物胶束化的影响。)或通过分析胶束化的摩尔吉布斯能量()。甲醇在水溶液中的影响表示为 和 Na +离子对两种热力学函数的作用相似: 和 。本文对胶束化的摩尔吉布斯能量进行了详细的分析: 和 以及它们的不同加载带来绝对错误。


























 京公网安备 11010802027423号
京公网安备 11010802027423号