当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Vapour-liquid equilibrium data for the carbon dioxide (CO2) + carbon monoxide (CO) system
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.jct.2020.106180 Antonin Chapoy , Pezhman Ahmadi , Valdério de Oliveira Cavalcanti Filho , Prashant Jadhawar
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.jct.2020.106180 Antonin Chapoy , Pezhman Ahmadi , Valdério de Oliveira Cavalcanti Filho , Prashant Jadhawar
|
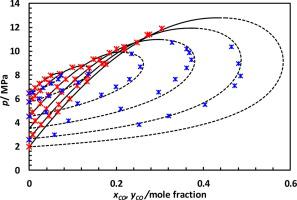
Abstract In carbon capture, utilization and storage (CCUS), a thorough understanding of thermophysical behaviour of the candidate fluid is an essential requirement for accurate design and optimised operation of the processes. In this communication, vapour liquid equilibrium data (VLE) of the binary mixtures of CO + CO2 are presented. A static-analytic method was used to obtain VLE data at six isotherms (253.15, 261.45, 273.00, 283.05, 293.05, 298.15) K and pressures up to 12 MPa. The standard uncertainties of the measured temperature and pressure were estimated to be 0.1 K, 0.005 MPa, respectively. Also, the standard uncertainty of the measured molar composition of each phase is found to be less than 1.1%. The measured experimental results are then compared with some predictive thermodynamic equations of state (EoS) (i.e. the Peng Robinson (PR-78) with classical or Wong-Sandler mixing rules, the GERG, and EoS-CG without and with a specific departure function) and available data in the literature. A sound agreement is observed between the results of this work and some of the VLE data published in the open literature. Furthermore, for all isotherms, the best agreement is observed between experimental results and predicted VLE data from the PR-EoS with the Wong-Sandler mixing rules and the EoS-CG with a specific departure function. However, a significant deviation is found between measured results and VLE data calculated using the GERG-EoS.
中文翻译:

二氧化碳 (CO2) + 一氧化碳 (CO) 系统的气液平衡数据
摘要 在碳捕获、利用和储存 (CCUS) 中,彻底了解候选流体的热物理行为是准确设计和优化过程操作的基本要求。在此通讯中,介绍了 CO + CO2 二元混合物的气液平衡数据 (VLE)。使用静态分析方法在六个等温线(253.15、261.45、273.00、283.05、293.05、298.15)K 和高达 12 MPa 的压力下获得 VLE 数据。测得的温度和压力的标准不确定度分别估计为 0.1 K、0.005 MPa。此外,发现每个相的测量摩尔组成的标准不确定度小于 1.1%。然后将测量的实验结果与一些预测的热力学状态方程 (EoS) 进行比较(即 Peng Robinson (PR-78) 与经典或 Wong-Sandler 混合规则,GERG 和 EoS-CG 没有和有特定的离场函数)和文献中的可用数据。在这项工作的结果与公开文献中发表的一些 VLE 数据之间观察到了良好的一致性。此外,对于所有等温线,在实验结果与来自具有 Wong-Sandler 混合规则的 PR-EoS 和具有特定偏离函数的 EoS-CG 的预测 VLE 数据之间观察到最佳一致性。然而,在测量结果和使用 GERG-EoS 计算的 VLE 数据之间发现了显着的偏差。在这项工作的结果与公开文献中发表的一些 VLE 数据之间观察到了良好的一致性。此外,对于所有等温线,在实验结果与来自具有 Wong-Sandler 混合规则的 PR-EoS 和具有特定偏离函数的 EoS-CG 的预测 VLE 数据之间观察到最佳一致性。然而,在测量结果和使用 GERG-EoS 计算的 VLE 数据之间发现了显着的偏差。在这项工作的结果与公开文献中发表的一些 VLE 数据之间观察到了良好的一致性。此外,对于所有等温线,在实验结果与来自具有 Wong-Sandler 混合规则的 PR-EoS 和具有特定偏离函数的 EoS-CG 的预测 VLE 数据之间观察到最佳一致性。然而,在测量结果和使用 GERG-EoS 计算的 VLE 数据之间发现了显着的偏差。
更新日期:2020-11-01
中文翻译:

二氧化碳 (CO2) + 一氧化碳 (CO) 系统的气液平衡数据
摘要 在碳捕获、利用和储存 (CCUS) 中,彻底了解候选流体的热物理行为是准确设计和优化过程操作的基本要求。在此通讯中,介绍了 CO + CO2 二元混合物的气液平衡数据 (VLE)。使用静态分析方法在六个等温线(253.15、261.45、273.00、283.05、293.05、298.15)K 和高达 12 MPa 的压力下获得 VLE 数据。测得的温度和压力的标准不确定度分别估计为 0.1 K、0.005 MPa。此外,发现每个相的测量摩尔组成的标准不确定度小于 1.1%。然后将测量的实验结果与一些预测的热力学状态方程 (EoS) 进行比较(即 Peng Robinson (PR-78) 与经典或 Wong-Sandler 混合规则,GERG 和 EoS-CG 没有和有特定的离场函数)和文献中的可用数据。在这项工作的结果与公开文献中发表的一些 VLE 数据之间观察到了良好的一致性。此外,对于所有等温线,在实验结果与来自具有 Wong-Sandler 混合规则的 PR-EoS 和具有特定偏离函数的 EoS-CG 的预测 VLE 数据之间观察到最佳一致性。然而,在测量结果和使用 GERG-EoS 计算的 VLE 数据之间发现了显着的偏差。在这项工作的结果与公开文献中发表的一些 VLE 数据之间观察到了良好的一致性。此外,对于所有等温线,在实验结果与来自具有 Wong-Sandler 混合规则的 PR-EoS 和具有特定偏离函数的 EoS-CG 的预测 VLE 数据之间观察到最佳一致性。然而,在测量结果和使用 GERG-EoS 计算的 VLE 数据之间发现了显着的偏差。在这项工作的结果与公开文献中发表的一些 VLE 数据之间观察到了良好的一致性。此外,对于所有等温线,在实验结果与来自具有 Wong-Sandler 混合规则的 PR-EoS 和具有特定偏离函数的 EoS-CG 的预测 VLE 数据之间观察到最佳一致性。然而,在测量结果和使用 GERG-EoS 计算的 VLE 数据之间发现了显着的偏差。



























 京公网安备 11010802027423号
京公网安备 11010802027423号