Solid State Ionics ( IF 3.2 ) Pub Date : 2020-05-30 , DOI: 10.1016/j.ssi.2020.115368 Qinyun Wang , Yanxiang Luo , FanPei Gu , Miao Shui , Jie Shu
|
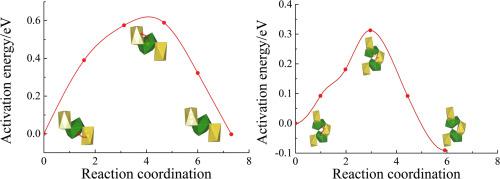
Due to the limited resources of lithium, sodium ion battery is regarded as the most promising alternative to lithium-ion batteries. In this study, the Cr coping and carbon coating anode material for SIBs, Na1.25Cr0.25Ti1.75(PO4)3/C, is synthesized by a facile sol-gel technique using polyvinyl alcohol as carbon source. The obtained products show remarkable electrochemical properties in rate-capability (148.6 mAh g−1 at 1C, 111.8 mAh g−1 at 2C, 91.2 mAh g−1 at 3C, 77.8 mAh g−1 at 4C, 68.3 mAh g−1 at 5C, 61.8 mAh g−1 at 6C, 57.4 mAh g−1 at 7C), cycle-stability (65.1% capacity retention over 1000 cycles) and Coulombic efficiencies. Furthermore, the doping of Cr element reduces the mean charge/discharge potential significantly and is favorable for the construction of the whole batteries with higher average potentials. The molecular dynamics simulations of the pristine NaTi2(PO4)3 and NaCr0.25Ti1.75(PO4)3 tells us that the Cr doping in the lattice increases the self-diffusion coefficients of Na+ by 12 orders of magnitude. This great enhancement of the Na+ mobility is supposed to be owing to the presence of the interstice Na+ and the concerted migration of interstice Na+ and the M1 site Na+, which owns a significantly reduced energy barrier compared with that of the vacancy migration mechanism in the case of pristine NaTi2(PO4)3.
中文翻译:

Na 1.25 Cr 0.25 Ti 1.75(PO 4)3 / C作为SIBs负极材料的制备,表征,电化学性能和迁移机理
由于锂的资源有限,钠离子电池被认为是锂离子电池最有希望的替代品。在这项研究中,SIBs的Cr涂层和碳涂层阳极材料Na 1.25 Cr 0.25 Ti 1.75(PO 4)3 / C是通过使用聚乙烯醇作为碳源的简便溶胶凝胶技术合成的。将得到的产品显示出速率能力(148.6毫安克显着的电化学特性-1以1C,111.8毫安克-1在图2C中,91.2毫安克-1在图3C中,77.8毫安克-1在4℃,68.3毫安克-1在如图5C所示,61.8毫安克-1在图6C中,57.4毫安克-1(在7C下),循环稳定性(在1000个循环中保持65.1%的容量)和库仑效率。此外,Cr元素的掺杂显着降低了平均充电/放电电势,并且有利于具有更高平均电势的整个电池的构造。原始的NaTi 2(PO 4)3和NaCr 0.25 Ti 1.75(PO 4)3的分子动力学模拟告诉我们,晶格中的Cr掺杂使Na +的自扩散系数增加了12个数量级。Na +迁移率的这种极大提高应该归因于存在空隙Na +以及间隙Na +和M1位点Na +的协同迁移,与原始NaTi 2(PO 4)3的空位迁移机理相比,它具有明显降低的能垒。


























 京公网安备 11010802027423号
京公网安备 11010802027423号