当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of the electronic state on low-rank coals with Ca2+ ion exchange
Journal of Molecular Structure ( IF 3.8 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.molstruc.2020.128544 Yuji Shinohara , Naoto Tsubouchi
Journal of Molecular Structure ( IF 3.8 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.molstruc.2020.128544 Yuji Shinohara , Naoto Tsubouchi
|
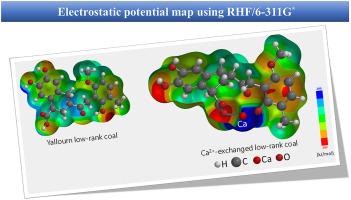
Abstract Ca2+-exchanged coal prepared from calcium hydroxide is a good catalyst for steam gasification of low-rank coal with a fixed-bed quartz reactor. However, it is difficult to clarify the effect of Ca2+ ion exchange on low-rank coal by experiment. To investigate the effect of Ca2+ ions on low-rank coals, this study determined the electronic states between Ca2+-exchanged and raw coal models and compared them by B3LYP/6-31G∗ and HF/6-311G∗ calculations. The experiments revealed that the Ca2+ ion exchange method significantly affects gasification of low-rank coal. It is considered that the molecular structure of low-rank coal presents significant changes in the electronic state before and after Ca exchange, even with a simple molecular structure. Therefore, a molecular model was created in which a COOH/OH H+ ion was exchanged with one Ca2+ ion, and quantum chemical calculations were subsequently performed. The results revealed that the –COOCa1/2 IR peak calculated from B3LYP/6-31G∗ appeared at approximately the same position as the experimental IR peak in low-rank coal after Ca exchange. The Ca structure was more stable following interaction with the O in the benzene OH group than with that in the OH group of the tetralin ring containing Ca. Furthermore, the increased negative charge of the benzene ring following Ca interaction was presumed to improve the reactivity between the low-rank coal and steam. In addition, weak bonds in the Ca2+-exchanged coal molecules were investigated by calculating the difference between the values of the Lowdin and Mulliken bond orders before and after Ca2+ exchange. The results indicated that the binding in the molecule was weakened by Ca coordination. From these facts, it is presumed that H+/Ca2+ ion exchange promotes the decomposition of low-rank coal.
中文翻译:

Ca2+离子交换电子态对低阶煤的影响
摘要 氢氧化钙制备的Ca2+交换煤是低阶煤用固定床石英反应器蒸汽气化的良好催化剂。然而,很难通过实验阐明Ca2+离子交换对低阶煤的影响。为了研究 Ca2+ 离子对低阶煤的影响,本研究确定了 Ca2+ 交换模型和原煤模型之间的电子状态,并通过 B3LYP/6-31G* 和 HF/6-311G* 计算进行了比较。实验表明,Ca2+离子交换法对低阶煤的气化有显着影响。认为即使分子结构简单,低阶煤的分子结构在Ca交换前后的电子态也发生了显着变化。因此,创建了一个分子模型,其中 COOH/OH H+ 离子与一个 Ca2+ 离子交换,随后进行了量子化学计算。结果表明,从 B3LYP/6-31G∗ 计算出的 –COOCa1/2 IR 峰出现在与 Ca 交换后低阶煤中的实验 IR 峰大致相同的位置。与苯 OH 基团中的 O 相互作用后,Ca 结构比与含 Ca 的四氢化萘环的 OH 基团中的 O 相互作用更稳定。此外,推测钙相互作用后苯环负电荷的增加可以提高低阶煤和蒸汽之间的反应性。此外,通过计算 Ca2+ 交换前后的 Lowdin 和 Mulliken 键级值之间的差异,研究了 Ca2+ 交换的煤分子中的弱键。结果表明,Ca 配位削弱了分子中的结合。
更新日期:2020-10-01
中文翻译:

Ca2+离子交换电子态对低阶煤的影响
摘要 氢氧化钙制备的Ca2+交换煤是低阶煤用固定床石英反应器蒸汽气化的良好催化剂。然而,很难通过实验阐明Ca2+离子交换对低阶煤的影响。为了研究 Ca2+ 离子对低阶煤的影响,本研究确定了 Ca2+ 交换模型和原煤模型之间的电子状态,并通过 B3LYP/6-31G* 和 HF/6-311G* 计算进行了比较。实验表明,Ca2+离子交换法对低阶煤的气化有显着影响。认为即使分子结构简单,低阶煤的分子结构在Ca交换前后的电子态也发生了显着变化。因此,创建了一个分子模型,其中 COOH/OH H+ 离子与一个 Ca2+ 离子交换,随后进行了量子化学计算。结果表明,从 B3LYP/6-31G∗ 计算出的 –COOCa1/2 IR 峰出现在与 Ca 交换后低阶煤中的实验 IR 峰大致相同的位置。与苯 OH 基团中的 O 相互作用后,Ca 结构比与含 Ca 的四氢化萘环的 OH 基团中的 O 相互作用更稳定。此外,推测钙相互作用后苯环负电荷的增加可以提高低阶煤和蒸汽之间的反应性。此外,通过计算 Ca2+ 交换前后的 Lowdin 和 Mulliken 键级值之间的差异,研究了 Ca2+ 交换的煤分子中的弱键。结果表明,Ca 配位削弱了分子中的结合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号