Journal of Allergy and Clinical Immunology ( IF 14.2 ) Pub Date : 2020-05-30 , DOI: 10.1016/j.jaci.2020.05.023 Franziska Roth-Walter 1 , Sheriene Moussa Afify 2 , Luis F Pacios 3 , Bart R Blokhuis 4 , Frank Redegeld 4 , Andreas Regner 5 , Lisa-Marie Petje 5 , Alessandro Fiocchi 6 , Eva Untersmayr 7 , Zdenek Dvorak 8 , Karin Hufnagl 1 , Isabella Pali-Schöll 1 , Erika Jensen-Jarolim 1
|
Background
Beta-lactoglobulin (BLG) is a bovine lipocalin in milk with an innate defense function. The circumstances under which BLG is associated with tolerance of or allergy to milk are not understood.
Objective
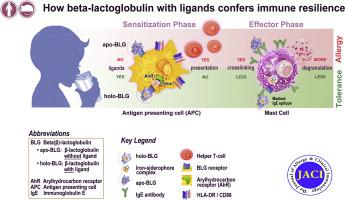
Our aims were to assess the capacity of ligand-free apoBLG versus loaded BLG (holoBLG) to protect mice against allergy by using an iron-quercetin complex as an exemplary ligand and to study the molecular mechanisms of this protection.
Methods
Binding of iron-quercetin to BLG was modeled and confirmed by spectroscopy and docking calculations. Serum IgE binding to apoBLG and holoBLG in children allergic to milk and children tolerant of milk was assessed. Mice were intranasally treated with apoBLG versus holoBLG and analyzed immunologically after systemic challenge. Aryl hydrocarbon receptor (AhR) activation was evaluated with reporter cells and Cyp1A1 expression. Treated human PBMCs and human mast cells were assessed by fluorescence-activated cell sorting and degranulation, respectively.
Results
Modeling predicted masking of major IgE and T-cell epitopes of BLG by ligand binding. In line with this modeling, IgE binding in children allergic to milk was reduced toward holoBLG, which also impaired degranulation of mast cells. In mice, only treatments with holoBLG prevented allergic sensitization and anaphylaxis, while sustaining regulatory T cells. BLG facilitated quercetin-dependent AhR activation and, downstream of AhR, lung Cyp1A1 expression. HoloBLG shuttled iron into monocytic cells and impaired their antigen presentation.
Conclusion
The cargo of holoBLG is decisive in preventing allergy in vivo. BLG without cargo acted as an allergen in vivo and further primed human mast cells for degranulation in an antigen-independent fashion. Our data provide a mechanistic explanation why the same proteins can act either as tolerogens or as allergens.
中文翻译:

牛奶蛋白β-乳球蛋白通过将复合铁靶向免疫细胞来赋予抗过敏能力。
背景
β-乳球蛋白(BLG)是牛奶中的牛脂蛋白,具有先天防御功能。不了解BLG与牛奶的耐受性或过敏相关的情况。
目的
我们的目标是通过使用铁槲皮素复合物作为示例性配体来评估无配体载脂蛋白BLG相对于负载BLG(holoBLG)保护小鼠免于过敏的能力,并研究这种保护的分子机制。
方法
对铁槲皮素与BLG的结合进行了建模,并通过光谱学和对接计算进行了证实。评估了对牛奶过敏的儿童和对牛奶耐受的儿童的血清IgE与apoBLG和holoBLG的结合。将小鼠用apoBLG与holoBLG鼻内治疗,并在全身性攻击后进行免疫学分析。使用报告细胞和Cyp1A1表达评估了芳烃受体(AhR)的活化。通过荧光激活细胞分选和脱颗粒分别评估处理过的人PBMC和人肥大细胞。
结果
通过配体结合建模预测BLG主要IgE和T细胞表位的掩蔽。与这种模型一致,对牛奶过敏的儿童中的IgE结合朝向holoBLG减少,这也削弱了肥大细胞的脱粒。在小鼠中,只有用holoBLG进行的治疗可以防止过敏性过敏和过敏反应,同时还能维持调节性T细胞。BLG促进槲皮素依赖性AhR激活,并在AhR下游促进肺Cyp1A1表达。HoloBLG将铁穿梭入单核细胞并破坏了其抗原呈递。
结论
holoBLG的货物在预防体内过敏方面起着决定性作用。没有货物的BLG在体内充当过敏原,并进一步以抗原非依赖性方式引发人类肥大细胞脱颗粒。我们的数据提供了一个机械的解释,为什么相同的蛋白质可以充当致耐受原或过敏原。


























 京公网安备 11010802027423号
京公网安备 11010802027423号