当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Combined KH/alkaline-earth metal amide catalysts for hydrogenation of alkenes
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-05-29 , DOI: 10.1039/d0qo00383b Xiang-Yu Zhang 1, 2, 3, 4, 5 , Hui-Zhen Du 1, 2, 3, 4, 5 , Dan-Dan Zhai 5, 6, 7, 8 , Bing-Tao Guan 5, 6, 7, 8
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-05-29 , DOI: 10.1039/d0qo00383b Xiang-Yu Zhang 1, 2, 3, 4, 5 , Hui-Zhen Du 1, 2, 3, 4, 5 , Dan-Dan Zhai 5, 6, 7, 8 , Bing-Tao Guan 5, 6, 7, 8
Affiliation
|
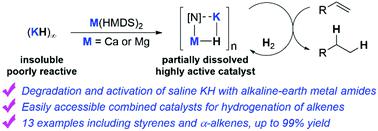
Using earth abundant and environmentally friendly base metal catalysts instead of noble transition metal catalysts is becoming a hot topic in catalytic hydrogenation reactions. Under this guidance, several soluble, structurally well-defined alkaline-earth metal hydride complexes were reported to catalyze hydrogenation reactions. The easily available alkali metal hydrides, however, showed poor activity probably because of their saline structures and high lattice energies. Herein, we reported the efficient hydrogenation of various styrenes and α-alkenes with the combined catalysts of KH and Ca(HMDS)2·(THF)2 or Mg(HMDS)2·Et2O (HMDS = −N(SiMe3)2). The combined catalysts exhibited much better activity than their components KH or alkaline-earth metal amides, suggesting an obvious bimetallic synergistic effect. Preliminary mechanism studies revealed a degradation and activation effect of the alkaline-earth metal amides on saline KH.
中文翻译:

KH /碱土金属酰胺组合催化剂用于烯烃加氢
在催化剂加氢反应中,使用对地球有利的环境友好的贱金属催化剂代替贵金属过渡金属催化剂已成为热门话题。在这种指导下,据报道有几种可溶性的,结构明确的碱土金属氢化物络合物可催化氢化反应。然而,容易获得的碱金属氢化物显示出较差的活性,这可能是由于其盐分结构和高晶格能量。本文中,我们报道了使用KH和Ca(HMDS)2 ·(THF)2或Mg(HMDS)2 ·Et 2 O(HMDS = -N(SiMe 3))的组合催化剂有效氢化各种苯乙烯和α-烯烃2)。组合的催化剂表现出比其组分KH或碱土金属酰胺更好的活性,表明明显的双金属协同作用。初步的机理研究表明,碱土金属酰胺对盐水KH的降解和活化作用。
更新日期:2020-07-28
中文翻译:

KH /碱土金属酰胺组合催化剂用于烯烃加氢
在催化剂加氢反应中,使用对地球有利的环境友好的贱金属催化剂代替贵金属过渡金属催化剂已成为热门话题。在这种指导下,据报道有几种可溶性的,结构明确的碱土金属氢化物络合物可催化氢化反应。然而,容易获得的碱金属氢化物显示出较差的活性,这可能是由于其盐分结构和高晶格能量。本文中,我们报道了使用KH和Ca(HMDS)2 ·(THF)2或Mg(HMDS)2 ·Et 2 O(HMDS = -N(SiMe 3))的组合催化剂有效氢化各种苯乙烯和α-烯烃2)。组合的催化剂表现出比其组分KH或碱土金属酰胺更好的活性,表明明显的双金属协同作用。初步的机理研究表明,碱土金属酰胺对盐水KH的降解和活化作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号