当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A theoretical investigation on the atmospheric degradation of the radical: reactions with NO, NO2, and NO3.
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2020-05-29 , DOI: 10.1039/d0em00112k Bo Feng 1 , Cuihong Sun , Weiwei Zhao , Shaowen Zhang
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2020-05-29 , DOI: 10.1039/d0em00112k Bo Feng 1 , Cuihong Sun , Weiwei Zhao , Shaowen Zhang
Affiliation
|
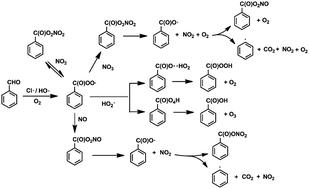
The 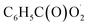 radical is the key intermediate in the atmospheric oxidation of benzaldehyde, and its further chemistry contributes to local air pollution. The reaction mechanisms of the
radical is the key intermediate in the atmospheric oxidation of benzaldehyde, and its further chemistry contributes to local air pollution. The reaction mechanisms of the 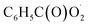 radical with NO, NO2,
radical with NO, NO2, 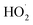 and NO3 were studied by quantum chemistry calculations at the CCSD(T)/CBS//M06-2X/def2-TZVP level of theory. The explicit potential energy curves were provided in order to reveal the atmospheric fate of the
and NO3 were studied by quantum chemistry calculations at the CCSD(T)/CBS//M06-2X/def2-TZVP level of theory. The explicit potential energy curves were provided in order to reveal the atmospheric fate of the 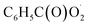 radical comprehensively. The main products of the reaction of
radical comprehensively. The main products of the reaction of 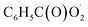 with NO are predicted to be
with NO are predicted to be 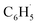 , CO2 and NO2. The reaction of
, CO2 and NO2. The reaction of 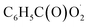 with NO2 is reversible, and its main product would be C6H5C(O)O2NO2 which was predicted to be more stable than PAN (peroxyacetyl nitrate) at room temperature. The decomposition of C6H5C(O)O2NO2 at different ambient temperatures would be a potential long-range transport source of NOx in the atmosphere. The predominant products of the reaction
with NO2 is reversible, and its main product would be C6H5C(O)O2NO2 which was predicted to be more stable than PAN (peroxyacetyl nitrate) at room temperature. The decomposition of C6H5C(O)O2NO2 at different ambient temperatures would be a potential long-range transport source of NOx in the atmosphere. The predominant products of the reaction 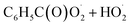 are predicted to be C6H5C(O)O2H, C6H5C(O)OH, O2 and O3, while HO˙ is of minor importance. So, the reaction of
are predicted to be C6H5C(O)O2H, C6H5C(O)OH, O2 and O3, while HO˙ is of minor importance. So, the reaction of 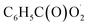 with
with 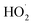 would be an important source of ozone and carboxylic acids in the local atmosphere, and has less contribution to the regeneration of HO˙ radicals. The reaction of
would be an important source of ozone and carboxylic acids in the local atmosphere, and has less contribution to the regeneration of HO˙ radicals. The reaction of 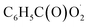 with NO3 should mainly produce
with NO3 should mainly produce 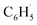 , CO2, O2 and NO2, which might play an important role in atmospheric chemistry of peroxy radicals at night, but has less contribution to the night-time conversion of
, CO2, O2 and NO2, which might play an important role in atmospheric chemistry of peroxy radicals at night, but has less contribution to the night-time conversion of 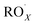 (
(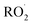 and RO˙) to
and RO˙) to 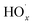 (
(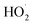 and HO˙) in the local atmosphere. The results above are in good accordance with the reported experimental observations.
and HO˙) in the local atmosphere. The results above are in good accordance with the reported experimental observations.
中文翻译:

对自由基在大气中降解的理论研究:与NO,NO2和NO3的反应。
该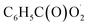 基团是关键在苯甲醛的大气氧化中间体,及其进一步的化学有助于当地的空气污染。所述的反应机理
基团是关键在苯甲醛的大气氧化中间体,及其进一步的化学有助于当地的空气污染。所述的反应机理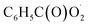 自由基与NO,NO 2,
自由基与NO,NO 2,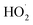 和NO 3均在CCSD(T)/ CBS // M06-2X / DEF2-TZVP理论水平研究了量子化学计算。提供了显式的势能曲线,以全面揭示
和NO 3均在CCSD(T)/ CBS // M06-2X / DEF2-TZVP理论水平研究了量子化学计算。提供了显式的势能曲线,以全面揭示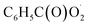 自由基的大气命运。
自由基的大气命运。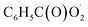 与NO反应的主要产物预计为
与NO反应的主要产物预计为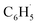 CO 2和NO 2。
CO 2和NO 2。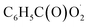 与NO 2的反应是可逆的,其主要产物为C 6 H预计在室温下比PAN(过氧乙酰硝酸盐)更稳定的5 C(O)O 2 NO 2。C 6 H 5 C(O)O 2 NO 2在不同环境温度下的分解将是大气中NO x的潜在长距离传输源。
与NO 2的反应是可逆的,其主要产物为C 6 H预计在室温下比PAN(过氧乙酰硝酸盐)更稳定的5 C(O)O 2 NO 2。C 6 H 5 C(O)O 2 NO 2在不同环境温度下的分解将是大气中NO x的潜在长距离传输源。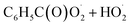 预计该反应的主要产物为C 6 H 5 C(O)O 2 H,C 6 H 5 C(O)OH,O 2和O 3,而HO 3的重要性较小。因此,反应
预计该反应的主要产物为C 6 H 5 C(O)O 2 H,C 6 H 5 C(O)OH,O 2和O 3,而HO 3的重要性较小。因此,反应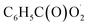 与
与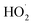 将是当地大气中臭氧和羧酸的重要来源,并且对HO +自由基再生的贡献较小。
将是当地大气中臭氧和羧酸的重要来源,并且对HO +自由基再生的贡献较小。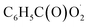 与NO 3的反应应主要产生
与NO 3的反应应主要产生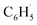 CO 2,O 2和NO 2,它们在夜间过氧自由基的大气化学中可能起重要作用,但对
CO 2,O 2和NO 2,它们在夜间过氧自由基的大气化学中可能起重要作用,但对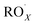 (
(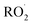 和RO˙)的夜间转化贡献较小。以
和RO˙)的夜间转化贡献较小。以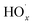 (
(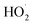 和浩)在当地的气氛。以上结果与所报道的实验观察结果非常吻合。
和浩)在当地的气氛。以上结果与所报道的实验观察结果非常吻合。
更新日期:2020-07-23
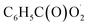 radical is the key intermediate in the atmospheric oxidation of benzaldehyde, and its further chemistry contributes to local air pollution. The reaction mechanisms of the
radical is the key intermediate in the atmospheric oxidation of benzaldehyde, and its further chemistry contributes to local air pollution. The reaction mechanisms of the 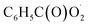 radical with NO, NO2,
radical with NO, NO2, 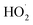 and NO3 were studied by quantum chemistry calculations at the CCSD(T)/CBS//M06-2X/def2-TZVP level of theory. The explicit potential energy curves were provided in order to reveal the atmospheric fate of the
and NO3 were studied by quantum chemistry calculations at the CCSD(T)/CBS//M06-2X/def2-TZVP level of theory. The explicit potential energy curves were provided in order to reveal the atmospheric fate of the 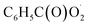 radical comprehensively. The main products of the reaction of
radical comprehensively. The main products of the reaction of 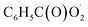 with NO are predicted to be
with NO are predicted to be 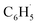 , CO2 and NO2. The reaction of
, CO2 and NO2. The reaction of 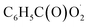 with NO2 is reversible, and its main product would be C6H5C(O)O2NO2 which was predicted to be more stable than PAN (peroxyacetyl nitrate) at room temperature. The decomposition of C6H5C(O)O2NO2 at different ambient temperatures would be a potential long-range transport source of NOx in the atmosphere. The predominant products of the reaction
with NO2 is reversible, and its main product would be C6H5C(O)O2NO2 which was predicted to be more stable than PAN (peroxyacetyl nitrate) at room temperature. The decomposition of C6H5C(O)O2NO2 at different ambient temperatures would be a potential long-range transport source of NOx in the atmosphere. The predominant products of the reaction 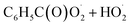 are predicted to be C6H5C(O)O2H, C6H5C(O)OH, O2 and O3, while HO˙ is of minor importance. So, the reaction of
are predicted to be C6H5C(O)O2H, C6H5C(O)OH, O2 and O3, while HO˙ is of minor importance. So, the reaction of 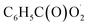 with
with 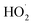 would be an important source of ozone and carboxylic acids in the local atmosphere, and has less contribution to the regeneration of HO˙ radicals. The reaction of
would be an important source of ozone and carboxylic acids in the local atmosphere, and has less contribution to the regeneration of HO˙ radicals. The reaction of 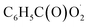 with NO3 should mainly produce
with NO3 should mainly produce 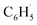 , CO2, O2 and NO2, which might play an important role in atmospheric chemistry of peroxy radicals at night, but has less contribution to the night-time conversion of
, CO2, O2 and NO2, which might play an important role in atmospheric chemistry of peroxy radicals at night, but has less contribution to the night-time conversion of 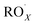 (
(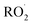 and RO˙) to
and RO˙) to 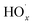 (
(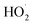 and HO˙) in the local atmosphere. The results above are in good accordance with the reported experimental observations.
and HO˙) in the local atmosphere. The results above are in good accordance with the reported experimental observations.
中文翻译:

对自由基在大气中降解的理论研究:与NO,NO2和NO3的反应。
该
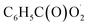 基团是关键在苯甲醛的大气氧化中间体,及其进一步的化学有助于当地的空气污染。所述的反应机理
基团是关键在苯甲醛的大气氧化中间体,及其进一步的化学有助于当地的空气污染。所述的反应机理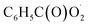 自由基与NO,NO 2,
自由基与NO,NO 2,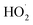 和NO 3均在CCSD(T)/ CBS // M06-2X / DEF2-TZVP理论水平研究了量子化学计算。提供了显式的势能曲线,以全面揭示
和NO 3均在CCSD(T)/ CBS // M06-2X / DEF2-TZVP理论水平研究了量子化学计算。提供了显式的势能曲线,以全面揭示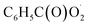 自由基的大气命运。
自由基的大气命运。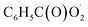 与NO反应的主要产物预计为
与NO反应的主要产物预计为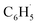 CO 2和NO 2。
CO 2和NO 2。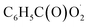 与NO 2的反应是可逆的,其主要产物为C 6 H预计在室温下比PAN(过氧乙酰硝酸盐)更稳定的5 C(O)O 2 NO 2。C 6 H 5 C(O)O 2 NO 2在不同环境温度下的分解将是大气中NO x的潜在长距离传输源。
与NO 2的反应是可逆的,其主要产物为C 6 H预计在室温下比PAN(过氧乙酰硝酸盐)更稳定的5 C(O)O 2 NO 2。C 6 H 5 C(O)O 2 NO 2在不同环境温度下的分解将是大气中NO x的潜在长距离传输源。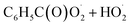 预计该反应的主要产物为C 6 H 5 C(O)O 2 H,C 6 H 5 C(O)OH,O 2和O 3,而HO 3的重要性较小。因此,反应
预计该反应的主要产物为C 6 H 5 C(O)O 2 H,C 6 H 5 C(O)OH,O 2和O 3,而HO 3的重要性较小。因此,反应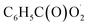 与
与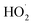 将是当地大气中臭氧和羧酸的重要来源,并且对HO +自由基再生的贡献较小。
将是当地大气中臭氧和羧酸的重要来源,并且对HO +自由基再生的贡献较小。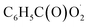 与NO 3的反应应主要产生
与NO 3的反应应主要产生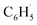 CO 2,O 2和NO 2,它们在夜间过氧自由基的大气化学中可能起重要作用,但对
CO 2,O 2和NO 2,它们在夜间过氧自由基的大气化学中可能起重要作用,但对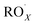 (
(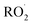 和RO˙)的夜间转化贡献较小。以
和RO˙)的夜间转化贡献较小。以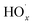 (
(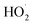 和浩)在当地的气氛。以上结果与所报道的实验观察结果非常吻合。
和浩)在当地的气氛。以上结果与所报道的实验观察结果非常吻合。


























 京公网安备 11010802027423号
京公网安备 11010802027423号