当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Cyclobutanation of Aryl Sulfonates through both C–O and C–H Cleavage
Organometallics ( IF 2.8 ) Pub Date : 2020-05-28 , DOI: 10.1021/acs.organomet.0c00338 Liangwei Zhang 1 , Long Liu 2 , Tianzeng Huang 2 , Qizhi Dong 1 , Tieqiao Chen 1, 2
Organometallics ( IF 2.8 ) Pub Date : 2020-05-28 , DOI: 10.1021/acs.organomet.0c00338 Liangwei Zhang 1 , Long Liu 2 , Tianzeng Huang 2 , Qizhi Dong 1 , Tieqiao Chen 1, 2
Affiliation
|
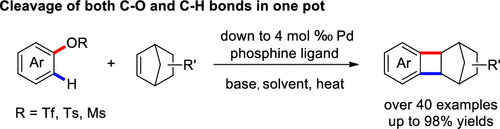
A palladium-catalyzed cyclobutanation of aryl sulfonates with strained alkenes has been developed. The methodology is featured to achieve the cleavage of both C–O and C–H bonds of phenol derivatives in one pot. Under the reaction conditions, in addition to reactive triflates, the relatively inactive tosylates and mesylates can also be transformed into the corresponding benzocyclobutanes in high yields. This reaction can be carried out in gram-scale experiments with a low loading of the palladium catalyst and is applicable to the cyclobutanative modification of bioactive molecules, highlighting its synthetic value in organic synthesis.
中文翻译:

通过C–O和C–H裂解进行钯催化的芳基磺酸盐环化
已经开发了钯催化的芳基磺酸盐与应变烯烃的环丁烷化反应。该方法的特点是可以在一锅中裂解酚衍生物的C–O和C–H键。在反应条件下,除了反应性三氟甲磺酸酯以外,还可以高产率将相对不活泼的甲苯磺酸酯和甲磺酸酯转化为相应的苯并环丁烷。该反应可以在克级实验中以低负载的钯催化剂进行,并且适用于生物活性分子的环丁酸酯化修饰,突出了其在有机合成中的合成价值。
更新日期:2020-05-28
中文翻译:

通过C–O和C–H裂解进行钯催化的芳基磺酸盐环化
已经开发了钯催化的芳基磺酸盐与应变烯烃的环丁烷化反应。该方法的特点是可以在一锅中裂解酚衍生物的C–O和C–H键。在反应条件下,除了反应性三氟甲磺酸酯以外,还可以高产率将相对不活泼的甲苯磺酸酯和甲磺酸酯转化为相应的苯并环丁烷。该反应可以在克级实验中以低负载的钯催化剂进行,并且适用于生物活性分子的环丁酸酯化修饰,突出了其在有机合成中的合成价值。



























 京公网安备 11010802027423号
京公网安备 11010802027423号