当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Efficient Synthesis of Tenofovir (PMPA): A Key Intermediate Leading to Tenofovir-Based HIV Medicines
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-05-29 , DOI: 10.1021/acs.oprd.0c00078 Brenden P. Derstine 1 , John W. Tomlin 1 , Cheryl L. Peck 1 , Jule-Phillip Dietz 2 , Brenden T. Herrera 1 , Flavio S. P. Cardoso 1 , Dinesh J. Paymode 1 , Andrew C. Yue 1 , Anthony J. Arduengo 3 , Till Opatz 2 , David R. Snead 1 , Rodger W. Stringham 1 , D. Tyler McQuade 1 , B. Frank Gupton 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-05-29 , DOI: 10.1021/acs.oprd.0c00078 Brenden P. Derstine 1 , John W. Tomlin 1 , Cheryl L. Peck 1 , Jule-Phillip Dietz 2 , Brenden T. Herrera 1 , Flavio S. P. Cardoso 1 , Dinesh J. Paymode 1 , Andrew C. Yue 1 , Anthony J. Arduengo 3 , Till Opatz 2 , David R. Snead 1 , Rodger W. Stringham 1 , D. Tyler McQuade 1 , B. Frank Gupton 1
Affiliation
|
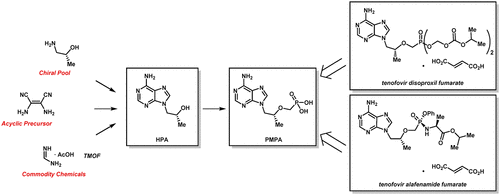
Herein, we report further improvements to the synthesis of tenofovir 1, the precursor to tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide fumarate (TAF). Starting from acyclic precursor diaminomalononitrile 12, a four-step protocol to tenofovir 1 will allow for vertical integration for more manufacturers. The key transformation is a convergent one-step procedure from 6 as compared to the current commercial process, with an improved yield from 59% (two steps) to 70%. Further improvements include eliminating the need for problematic magnesium tert-butoxide (MTB) and significant solvent reduction by avoiding an intermediate workup. With the costs of HIV/AIDS treatments remaining a barrier for those most in need, lowering the raw material/processing costs and increasing the security of supply can increase patient access.
中文翻译:

替诺福韦(PMPA)的有效合成:导致基于替诺福韦的HIV药物的关键中间体
在本文中,我们报告了对替诺福韦1(替诺福韦二富马酸富马酸酯(TDF)和替诺福韦阿拉芬酰胺富马酸酯(TAF)的前体)合成的进一步改进。从无环前体二氨基丙二腈12开始,到替诺福韦1的四步方案将允许更多制造商进行垂直整合。与当前的商业流程相比,关键的转变是从6开始的收敛的一步式过程,产率从59%(两步)提高到70%。进一步的改进包括消除有问题的镁叔叔的需求-丁醇盐(MTB),并通过避免中间处理显着减少溶剂。由于艾滋病毒/艾滋病治疗的费用仍然是最需要帮助的人的障碍,因此降低原材料/加工成本和增加供应安全性可以增加患者的出诊率。
更新日期:2020-05-29
中文翻译:

替诺福韦(PMPA)的有效合成:导致基于替诺福韦的HIV药物的关键中间体
在本文中,我们报告了对替诺福韦1(替诺福韦二富马酸富马酸酯(TDF)和替诺福韦阿拉芬酰胺富马酸酯(TAF)的前体)合成的进一步改进。从无环前体二氨基丙二腈12开始,到替诺福韦1的四步方案将允许更多制造商进行垂直整合。与当前的商业流程相比,关键的转变是从6开始的收敛的一步式过程,产率从59%(两步)提高到70%。进一步的改进包括消除有问题的镁叔叔的需求-丁醇盐(MTB),并通过避免中间处理显着减少溶剂。由于艾滋病毒/艾滋病治疗的费用仍然是最需要帮助的人的障碍,因此降低原材料/加工成本和增加供应安全性可以增加患者的出诊率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号