当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility Advantage of Amorphous Ketoprofen. Thermodynamic and Kinetic Aspects by Molecular Dynamics and Free Energy Approaches.
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2020-05-28 , DOI: 10.1021/acs.jctc.0c00166 D Gobbo 1 , P Ballone 1, 2, 3 , S Decherchi 1 , A Cavalli 1, 4
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2020-05-28 , DOI: 10.1021/acs.jctc.0c00166 D Gobbo 1 , P Ballone 1, 2, 3 , S Decherchi 1 , A Cavalli 1, 4
Affiliation
|
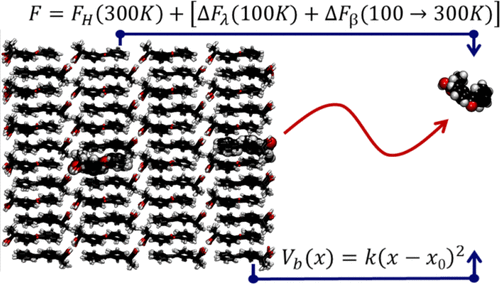
Thermodynamic and kinetic aspects of crystalline (c-KTP) and amorphous (a-KTP) ketoprofen dissolution in water have been investigated by molecular dynamics simulation focusing on free energy properties. Absolute free energies of all relevant species and phases have been determined by thermodynamic integration on a novel path, first connecting the harmonic to the anharmonic system Hamiltonian at low T and then extending the result to the temperature of interest. The free energy required to transfer one ketoprofen molecule from the crystal to the solution is in fair agreement with the experimental value. The absolute free energy of the amorphous form is 19.58 kJ/mol higher than for the crystal, greatly enhancing the ketoprofen concentration in water, although as a metastable species in supersaturated solution. The kinetics of the dissolution process has been analyzed by computing the free energy profile along a reaction coordinate bringing one ketoprofen molecule from the crystal or amorphous phase to the solvated state. This computation confirms that, compared to the crystal form, the dissolution rate is nearly 7 orders of magnitude faster for the amorphous form, providing one further advantage to the latter in terms of bioavailability. The problem of drug solubility, of great practical importance, is used here as a test bed for a refined method to compute absolute free energies, which could be of great interest in biophysics and drug discovery in particular.
中文翻译:

非晶态酮洛芬的溶解度优势。通过分子动力学和自由能方法的热力学和动力学方面。
通过关注自由能特性的分子动力学模拟研究了晶体(c-KTP)和无定形(a-KTP)酮洛芬在水中的热力学和动力学方面。已经通过一条新颖的路径上的热力学积分确定了所有相关物种和相的绝对自由能,首先将谐波与低T下的哈密顿系统耦合然后将结果扩展到目标温度。将一个酮洛芬分子从晶体转移到溶液中所需的自由能与实验值完全一致。非晶态的绝对自由能比晶体的绝对自由能高19.58 kJ / mol,尽管在过饱和溶液中是亚稳态物种,但大大提高了水中酮洛芬的浓度。通过沿着反应坐标计算自由能曲线来分析溶解过程的动力学,该反应坐标使一个酮洛芬分子从晶体或无定形状态转变为溶剂化状态。该计算证实,与晶体形式相比,无定形形式的溶解速率快近7个数量级,在生物利用度方面为后者提供了另一优点。
更新日期:2020-07-14
中文翻译:

非晶态酮洛芬的溶解度优势。通过分子动力学和自由能方法的热力学和动力学方面。
通过关注自由能特性的分子动力学模拟研究了晶体(c-KTP)和无定形(a-KTP)酮洛芬在水中的热力学和动力学方面。已经通过一条新颖的路径上的热力学积分确定了所有相关物种和相的绝对自由能,首先将谐波与低T下的哈密顿系统耦合然后将结果扩展到目标温度。将一个酮洛芬分子从晶体转移到溶液中所需的自由能与实验值完全一致。非晶态的绝对自由能比晶体的绝对自由能高19.58 kJ / mol,尽管在过饱和溶液中是亚稳态物种,但大大提高了水中酮洛芬的浓度。通过沿着反应坐标计算自由能曲线来分析溶解过程的动力学,该反应坐标使一个酮洛芬分子从晶体或无定形状态转变为溶剂化状态。该计算证实,与晶体形式相比,无定形形式的溶解速率快近7个数量级,在生物利用度方面为后者提供了另一优点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号