当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deactivation Kinetics of the Catalytic Alkylation Reaction
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-05-29 , DOI: 10.1021/acscatal.0c00932 Aditya Sengar 1 , Rutger A. van Santen 1, 2 , Johannes A.M. Kuipers 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-05-29 , DOI: 10.1021/acscatal.0c00932 Aditya Sengar 1 , Rutger A. van Santen 1, 2 , Johannes A.M. Kuipers 1
Affiliation
|
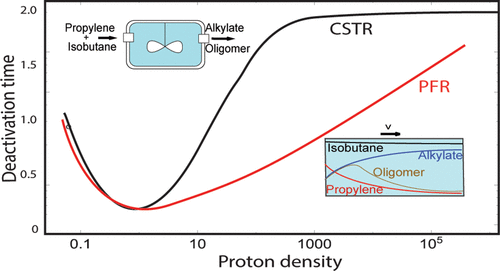
A kinetics theory of catalyst deactivation is presented of the solid acid-catalyzed alkylation reaction of isobutane with propylene or butene that gives alkylate, a high octane fuel, as product. The intimate relation between the kinetics network of the reaction, catalyst deactivation kinetics, and residence time distribution is analyzed. The question is addressed why the deactivation of the alkylation reaction in a continuously stirred tank reactor (CSTR) is slow compared to that in a tubular plug flow reactor (PFR). Conditions are derived where such differences will be minimum and maximum. In the reaction regime of high alkylate selectivity, linear and quadratic power law kinetics equations in propylene concentration can be deduced from microkinetics. They are used to derive analytical expressions of deactivation times for CSTR and PFR. The theoretical power law kinetics equations can be related to previously established empirical rate equations of catalyst deactivation. We show that, in the CSTR, the self-alkylation reaction path contributes substantially to the deactivation time. In the self-alkylation reaction, alkylate is formed by reaction of the isobutene reaction intermediate and isobutane. Catalysts of high proton strength can benefit catalyst deactivation times by suppressing the carbenium ion deprotonation reaction that produces alkenes as isobutene. In the PFR, selective alkylate formation occurs only when the reaction occurs in a reaction zone of the catalyst bed. Deactivation is faster than in CSTR because of the reactant profile in the reaction zone. This reaction zone has restricted mobility due to the fast deactivation of reactive protons located behind the reaction zone by alkenes formed by nonselective reactions in the reaction zone. In PFR, as long as the reaction is limited to an immobile reaction zone, deactivation time is independent of reaction site density and contact time. Contact time dependence arises when the reaction zone is mobile. Overall deactivation time then depends strongly on the degree of deactivation of the protons behind the reaction zone.
中文翻译:

催化烷基化反应的失活动力学
提出了催化剂失活的动力学理论,即固体酸催化的异丁烷与丙烯或丁烯的烷基化反应,生成的烷基化产物为高辛烷值燃料。分析了反应动力学网络,催化剂失活动力学和停留时间分布之间的密切关系。解决了这个问题,为什么与连续活塞流反应器(PFR)相比,连续搅拌釜反应器(CSTR)中的烷基化反应失活缓慢。得出条件,其中这种差异将是最小和最大。在高烷基化选择性的反应方案中,可以从微观动力学推导丙烯浓度的线性和二次幂定律动力学方程。它们用于导出CSTR和PFR失活时间的解析表达式。理论功率定律动力学方程可以与先前建立的催化剂失活的经验速率方程相关。我们表明,在CSTR中,自烷基化反应路径对失活时间有很大贡献。在自烷基化反应中,通过异丁烯反应中间体和异丁烷的反应形成烷基化物。高质子强度的催化剂可通过抑制产生作为异丁烯的烯烃的碳鎓离子去质子化反应,从而缩短催化剂失活时间。在PFR中,仅当反应在催化剂床的反应区中发生时才发生选择性烷基化物的形成。由于反应区中的反应物分布,失活比CSTR更快。由于位于反应区后面的反应性质子被反应区中非选择性反应形成的烯烃迅速失活,因此该反应区的迁移率受到限制。在PFR中,只要反应限于不动的反应区,失活时间与反应位点密度和接触时间无关。当反应区是可移动的时,就会产生接触时间依赖性。然后,总的失活时间在很大程度上取决于反应区后面的质子的失活程度。
更新日期:2020-07-02
中文翻译:

催化烷基化反应的失活动力学
提出了催化剂失活的动力学理论,即固体酸催化的异丁烷与丙烯或丁烯的烷基化反应,生成的烷基化产物为高辛烷值燃料。分析了反应动力学网络,催化剂失活动力学和停留时间分布之间的密切关系。解决了这个问题,为什么与连续活塞流反应器(PFR)相比,连续搅拌釜反应器(CSTR)中的烷基化反应失活缓慢。得出条件,其中这种差异将是最小和最大。在高烷基化选择性的反应方案中,可以从微观动力学推导丙烯浓度的线性和二次幂定律动力学方程。它们用于导出CSTR和PFR失活时间的解析表达式。理论功率定律动力学方程可以与先前建立的催化剂失活的经验速率方程相关。我们表明,在CSTR中,自烷基化反应路径对失活时间有很大贡献。在自烷基化反应中,通过异丁烯反应中间体和异丁烷的反应形成烷基化物。高质子强度的催化剂可通过抑制产生作为异丁烯的烯烃的碳鎓离子去质子化反应,从而缩短催化剂失活时间。在PFR中,仅当反应在催化剂床的反应区中发生时才发生选择性烷基化物的形成。由于反应区中的反应物分布,失活比CSTR更快。由于位于反应区后面的反应性质子被反应区中非选择性反应形成的烯烃迅速失活,因此该反应区的迁移率受到限制。在PFR中,只要反应限于不动的反应区,失活时间与反应位点密度和接触时间无关。当反应区是可移动的时,就会产生接触时间依赖性。然后,总的失活时间在很大程度上取决于反应区后面的质子的失活程度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号