Cell ( IF 64.5 ) Pub Date : 2020-05-29 , DOI: 10.1016/j.cell.2020.02.003 Ping-Pui Wong 1 , José M Muñoz-Félix 2 , Maruan Hijazi 3 , Hyojin Kim 4 , Stephen D Robinson 5 , Beatriz De Luxán-Delgado 2 , Irene Rodríguez-Hernández 6 , Oscar Maiques 6 , Ya-Ming Meng 7 , Qiong Meng 7 , Natalia Bodrug 2 , Matthew Scott Dukinfield 2 , Louise E Reynolds 2 , George Elia 2 , Andrew Clear 3 , Catherine Harwood 8 , Yu Wang 9 , James J Campbell 9 , Rajinder Singh 9 , Penglie Zhang 9 , Thomas J Schall 9 , Kylie P Matchett 10 , Neil C Henderson 10 , Peter W Szlosarek 11 , Sally A Dreger 2 , Sally Smith 2 , J Louise Jones 2 , John G Gribben 3 , Pedro R Cutillas 3 , Pascal Meier 4 , Victoria Sanz-Moreno 6 , Kairbaan M Hodivala-Dilke 2
|
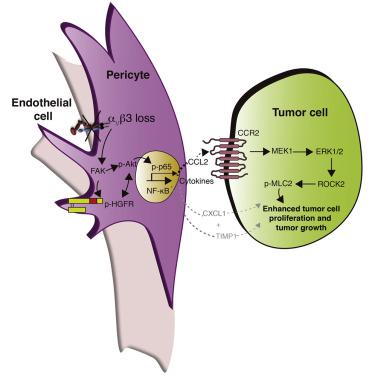
Enhanced blood vessel (BV) formation is thought to drive tumor growth through elevated nutrient delivery. However, this observation has overlooked potential roles for mural cells in directly affecting tumor growth independent of BV function. Here we provide clinical data correlating high percentages of mural-β3-integrin-negative tumor BVs with increased tumor sizes but no effect on BV numbers. Mural-β3-integrin loss also enhances tumor growth in implanted and autochthonous mouse tumor models with no detectable effects on BV numbers or function. At a molecular level, mural-cell β3-integrin loss enhances signaling via FAK-p-HGFR-p-Akt-p-p65, driving CXCL1, CCL2, and TIMP-1 production. In particular, mural-cell-derived CCL2 stimulates tumor cell MEK1-ERK1/2-ROCK2-dependent signaling and enhances tumor cell survival and tumor growth. Overall, our data indicate that mural cells can control tumor growth via paracrine signals regulated by β3-integrin, providing a previously unrecognized mechanism of cancer growth control.
中文翻译:

癌症负担由壁细胞-β3-整合素与肿瘤细胞的串扰控制。
增强的血管(BV)形成被认为可以通过增加营养输送来驱动肿瘤生长。然而,这一观察忽略了壁细胞在不依赖 BV 功能的情况下直接影响肿瘤生长的潜在作用。在这里,我们提供了高比例的壁-β3-整合素阴性肿瘤 BV 与肿瘤大小增加相关的临床数据,但对 BV 数量没有影响。壁-β3-整合素丢失也会促进植入和原产小鼠肿瘤模型中的肿瘤生长,但对 BV 数量或功能没有可检测到的影响。在分子水平上,壁细胞 β3-整合素丢失增强了通过 FAK-p-HGFR-p-Akt-p-p65 的信号传导,驱动 CXCL1、CCL2 和 TIMP-1 的产生。特别是,壁细胞衍生的 CCL2 刺激肿瘤细胞 MEK1-ERK1/2-ROCK2 依赖性信号传导,增强肿瘤细胞存活和肿瘤生长。总的来说,我们的数据表明壁细胞可以通过β3-整合素调节的旁分泌信号来控制肿瘤生长,提供了一种以前未被认识的癌症生长控制机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号