Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 3.2 ) Pub Date : 2020-05-29 , DOI: 10.1016/j.bbapap.2020.140459 Tomoki Nakayoshi 1 , Koichi Kato 2 , Shuichi Fukuyoshi 3 , Hiro Takahashi 3 , Ohgi Takahashi 4 , Eiji Kurimoto 5 , Akifumi Oda 6
|
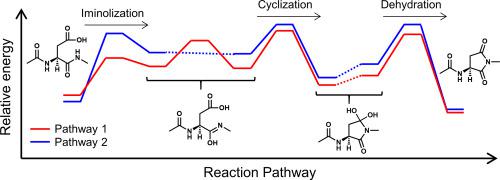
In the biological proteins, aspartic acid (Asp) residues are prone to nonenzymatic isomerization via a succinimide (Suc) intermediate. Asp-residue isomerization causes the aggregation and the insolubilization of proteins, and is considered to be involved in various age-related diseases. Although Suc intermediate was considered to be formed by nucleophilic attack of the main-chain amide nitrogen of N-terminal side adjacent residue to the side-chain carboxyl carbon of Asp residue, previous studies have shown that the nucleophilic attack is more likely to proceed via iminol tautomer when the water molecules act as catalysts. However, the full pathway to Suc-intermediate formation has not been investigated, and the experimental analyses for the Asp-residue isomerization mechanism at atomic and molecular levels, such as the analysis of the transition state geometry, are difficult. In the present study, we computationally explored the full pathways for Suc-intermediate formation from Asp residues. The calculations were performed two types of reactant complexes, and all energy minima and TS geometries were optimized using B3LYP density functional methods. As a result, the SI-intermediate formation was divided into three processes, i.e., iminolization, cyclization, and dehydration processes, and the activation energies were calculated to be 26.1 or 28.4 kcal mol−1. These values reproduce the experimental data. The computational results show that abundant water molecules in living organisms are effective catalysts for the Asp-residue isomerization.
中文翻译:

两个水分子催化的天冬氨酸残基非酶促琥珀酰亚胺形成机理的计算研究。
在生物蛋白质中,天冬氨酸(Asp)残基易于通过琥珀酰亚胺(Suc)中间体进行非酶异构化。天冬氨酸残基的异构化导致蛋白质的聚集和不溶,被认为与多种年龄相关疾病有关。尽管认为Suc中间体是由N末端侧相邻残基的主链酰胺氮对Asp残基的侧链羧基碳的亲核攻击形成的,但先前的研究表明,亲核攻击更可能通过水分子充当催化剂时的亚氨基互变异构体。但是,尚未研究形成Suc中间体的完整途径,并且在原子和分子水平对Asp残基异构化机理进行了实验分析,例如分析过渡态的几何形状,非常困难。在本研究中,我们通过计算探索了从Asp残基形成Suc中间体的完整途径。进行了两种类型的反应物络合物的计算,并使用B3LYP密度泛函方法优化了所有的能量最小值和TS几何形状。结果,SI-中间体的形成分为亚氨基化,环化和脱水三个过程,活化能经计算为26.1或28.4 kcal mol。并且使用B3LYP密度泛函方法优化了所有的最小能量和TS几何形状。结果,SI-中间体的形成分为亚氨基化,环化和脱水三个过程,活化能经计算为26.1或28.4 kcal mol。并且使用B3LYP密度泛函方法优化了所有的最小能量和TS几何形状。结果,SI-中间体的形成分为亚氨基化,环化和脱水三个过程,活化能经计算为26.1或28.4 kcal mol。-1。这些值再现了实验数据。计算结果表明,活生物体中丰富的水分子是有效的Asp残基异构化催化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号