当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Model for the Analysis of Membrane-Type Dissolution Tests for Inhaled Drugs.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-05-28 , DOI: 10.1021/acs.molpharmaceut.0c00163 Göran Frenning 1 , Irès van der Zwaan 1 , Frans Franek 2 , Rebecca Fransson 3 , Ulrika Tehler 3
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-05-28 , DOI: 10.1021/acs.molpharmaceut.0c00163 Göran Frenning 1 , Irès van der Zwaan 1 , Frans Franek 2 , Rebecca Fransson 3 , Ulrika Tehler 3
Affiliation
|
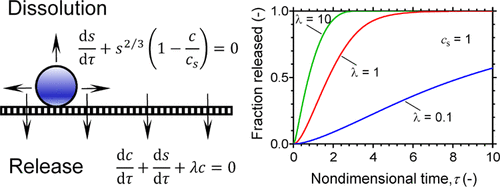
Impactor-type dose deposition is a common prerequisite for dissolution testing of inhaled medicines, and drug release typically takes place through a membrane. The purpose of this work is to develop a mechanistic model for such combined dissolution and release processes, focusing on a drug that initially is present in solid form. Our starting points are the Noyes–Whitney (or Nernst–Brunner) equation and Fick’s law. A detailed mechanistic analysis of the drug release process is provided, and approximate closed-form expressions for the amount of the drug that remains in solid form and the amount of the drug that has been released are derived. Comparisons with numerical data demonstrated the accuracy of the approximate expressions. Comparisons with experimental release data from literature demonstrated that the model can be used to establish rate-controlling release mechanisms. In conclusion, the model constitutes a valuable tool for the analysis of in vitro dissolution data for inhaled drugs.
中文翻译:

吸入药物的膜型溶出度分析的模型。
冲击器式剂量沉积是吸入药物溶出度测试的常见先决条件,药物释放通常通过膜发生。这项工作的目的是为这种结合的溶解和释放过程开发一种机械模型,重点是最初以固体形式存在的药物。我们的出发点是Noyes–Whitney(或Nernst–Brunner)方程式和Fick定律。提供了药物释放过程的详细机理分析,并得出了保留为固体形式的药物量和已释放的药物量的近似闭式表达式。与数值数据的比较证明了近似表达式的准确性。与来自文献的实验释放数据的比较表明,该模型可用于建立速率控制释放机制。总之,该模型是用于分析吸入药物的体外溶出度数据的有价值的工具。
更新日期:2020-07-06
中文翻译:

吸入药物的膜型溶出度分析的模型。
冲击器式剂量沉积是吸入药物溶出度测试的常见先决条件,药物释放通常通过膜发生。这项工作的目的是为这种结合的溶解和释放过程开发一种机械模型,重点是最初以固体形式存在的药物。我们的出发点是Noyes–Whitney(或Nernst–Brunner)方程式和Fick定律。提供了药物释放过程的详细机理分析,并得出了保留为固体形式的药物量和已释放的药物量的近似闭式表达式。与数值数据的比较证明了近似表达式的准确性。与来自文献的实验释放数据的比较表明,该模型可用于建立速率控制释放机制。总之,该模型是用于分析吸入药物的体外溶出度数据的有价值的工具。

























 京公网安备 11010802027423号
京公网安备 11010802027423号