当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fragment-Based Discovery of Pyrazolopyridones as JAK1 Inhibitors with Excellent Subtype Selectivity.
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-05-28 , DOI: 10.1021/acs.jmedchem.0c00359 Bettina Borreschmidt Hansen , Tue Heesgaard Jepsen , Mogens Larsen , Rikke Sindet , Thomas Vifian , Mia Nørreskov Burhardt , Jens Larsen , Jimmi Gerner Seitzberg , Martin A Carnerup , Anders Jerre , Christina Mølck , Paola Lovato , Sanjay Rai 1 , Venkatarathnam Reddy Nasipireddy 1 , Andreas Ritzén
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-05-28 , DOI: 10.1021/acs.jmedchem.0c00359 Bettina Borreschmidt Hansen , Tue Heesgaard Jepsen , Mogens Larsen , Rikke Sindet , Thomas Vifian , Mia Nørreskov Burhardt , Jens Larsen , Jimmi Gerner Seitzberg , Martin A Carnerup , Anders Jerre , Christina Mølck , Paola Lovato , Sanjay Rai 1 , Venkatarathnam Reddy Nasipireddy 1 , Andreas Ritzén
Affiliation
|
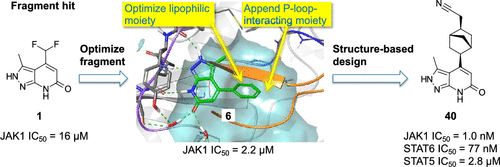
Herein, we report the discovery of a series of JAK1-selective kinase inhibitors with high potency and excellent JAK family subtype selectivity. A fragment screening hit 1 with a pyrazolopyridone core and a JAK1 bias was selected as the starting point for our fragment-based lead generation efforts. A two-stage strategy was chosen with the dual aims of improving potency and JAK1 selectivity: Optimization of the lipophilic ribose pocket-targeting substituent was followed by the introduction of a variety of P-loop-targeting functional groups. Combining the best moieties from both stages of the optimization afforded compound 40, which showed excellent potency and selectivity. Metabolism studies in vitro and in vivo together with an in vitro safety evaluation suggest that 40 may be a viable lead compound for the development of highly subtype-selective JAK1 inhibitors.
中文翻译:

基于片段的吡唑并吡啶酮作为JAK1抑制剂的发现,具有出色的亚型选择性。
在这里,我们报告发现了一系列具有高效力和出色的JAK家族亚型选择性的JAK1选择性激酶抑制剂。选择具有吡唑并吡啶酮核心和JAK1偏倚的片段筛选结果1作为我们基于片段的线索生成工作的起点。选择了两阶段策略,以提高效能和JAK1选择性的双重目的:优化亲脂性核糖口袋靶向的取代基,然后引入各种靶向P环的官能团。结合来自优化的两个阶段的最佳部分,得到化合物40,其显示出优异的效能和选择性。代谢研究在体外和体内具有一起体外安全性评估表明40可能是开发高度亚型选择性JAK1抑制剂的可行先导化合物。
更新日期:2020-07-09
中文翻译:

基于片段的吡唑并吡啶酮作为JAK1抑制剂的发现,具有出色的亚型选择性。
在这里,我们报告发现了一系列具有高效力和出色的JAK家族亚型选择性的JAK1选择性激酶抑制剂。选择具有吡唑并吡啶酮核心和JAK1偏倚的片段筛选结果1作为我们基于片段的线索生成工作的起点。选择了两阶段策略,以提高效能和JAK1选择性的双重目的:优化亲脂性核糖口袋靶向的取代基,然后引入各种靶向P环的官能团。结合来自优化的两个阶段的最佳部分,得到化合物40,其显示出优异的效能和选择性。代谢研究在体外和体内具有一起体外安全性评估表明40可能是开发高度亚型选择性JAK1抑制剂的可行先导化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号