当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coupled Copper-Zinc Catalysts for Electrochemical Reduction of Carbon Dioxide.
ChemSusChem ( IF 8.4 ) Pub Date : 2020-05-28 , DOI: 10.1002/cssc.202000971 Juqin Zeng 1 , Telemaco Rino 1, 2 , Katarzyna Bejtka 1 , Micaela Castellino 2 , Adriano Sacco 1 , M Amin Farkhondehfal 1 , Angelica Chiodoni 1 , Filippo Drago 3 , Candido F Pirri 1, 2
ChemSusChem ( IF 8.4 ) Pub Date : 2020-05-28 , DOI: 10.1002/cssc.202000971 Juqin Zeng 1 , Telemaco Rino 1, 2 , Katarzyna Bejtka 1 , Micaela Castellino 2 , Adriano Sacco 1 , M Amin Farkhondehfal 1 , Angelica Chiodoni 1 , Filippo Drago 3 , Candido F Pirri 1, 2
Affiliation
|
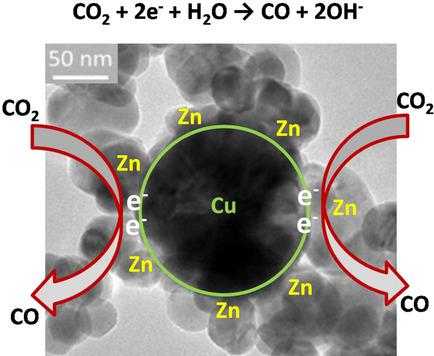
A catalyst plays a key role in the electrochemical reduction of CO2 to valuable chemicals and fuels. Hence, the development of efficient and inexpensive catalysts has attracted great interest from both the academic and industrial communities. In this work, low‐cost catalysts coupling Cu and Zn are designed and prepared with a green microwave‐assisted route. The Cu to Zn ratio in the catalysts can be easily tuned by adjusting the precursor solutions. The obtained Cu–Zn catalysts are mainly composed of polycrystalline Cu particles and monocrystalline ZnO nanoparticles. The electrodes with optimized Cu–Zn catalysts show enhanced CO production rates of approximately 200 μmol h−1 cm−2 with respect to those with a monometallic Cu or ZnO catalyst under the same applied potential. At the bimetallic electrodes, ZnO‐derived active sites are selective for CO formation and highly conductive Cu favors electron transport in the catalyst layer as well as charge transfer at the electrode/electrolyte interface.
中文翻译:

用于电化学还原二氧化碳的铜-锌偶联催化剂。
催化剂在将CO 2电化学还原成有价值的化学物质和燃料中起关键作用。因此,开发高效和廉价的催化剂引起了学术界和工业界的极大兴趣。在这项工作中,采用绿色微波辅助路线设计和制备了偶联铜和锌的低成本催化剂。通过调节前体溶液,可以轻松地调节催化剂中的铜锌比。所获得的Cu-Zn催化剂主要由多晶Cu颗粒和单晶ZnO纳米颗粒组成。使用优化的Cu-Zn催化剂的电极显示出更高的CO生成速率,约为200μmolh -1 cm -2对于那些在相同的施加电势下具有单金属Cu或ZnO催化剂的催化剂而言。在双金属电极上,ZnO衍生的活性位点对于形成CO具有选择性,而高导电性的Cu有利于催化剂层中的电子传输以及电极/电解质界面处的电荷转移。
更新日期:2020-05-28
中文翻译:

用于电化学还原二氧化碳的铜-锌偶联催化剂。
催化剂在将CO 2电化学还原成有价值的化学物质和燃料中起关键作用。因此,开发高效和廉价的催化剂引起了学术界和工业界的极大兴趣。在这项工作中,采用绿色微波辅助路线设计和制备了偶联铜和锌的低成本催化剂。通过调节前体溶液,可以轻松地调节催化剂中的铜锌比。所获得的Cu-Zn催化剂主要由多晶Cu颗粒和单晶ZnO纳米颗粒组成。使用优化的Cu-Zn催化剂的电极显示出更高的CO生成速率,约为200μmolh -1 cm -2对于那些在相同的施加电势下具有单金属Cu或ZnO催化剂的催化剂而言。在双金属电极上,ZnO衍生的活性位点对于形成CO具有选择性,而高导电性的Cu有利于催化剂层中的电子传输以及电极/电解质界面处的电荷转移。


























 京公网安备 11010802027423号
京公网安备 11010802027423号