当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel, Self-Distinguished, Dual Stimulus-Responsive Therapeutic Nanoplatform for Intracellular On-Demand Drug Release.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-05-27 , DOI: 10.1021/acs.molpharmaceut.0c00165 Heng Sun 1 , Zhongxiong Fan 1 , Sijin Xiang 2 , Wenbao Zuo 3 , Yifan Yang 1 , Doudou Huang 4 , Guanghao Su 5 , Xu Fu 6 , Qingliang Zhao 4 , Zhenqing Hou 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-05-27 , DOI: 10.1021/acs.molpharmaceut.0c00165 Heng Sun 1 , Zhongxiong Fan 1 , Sijin Xiang 2 , Wenbao Zuo 3 , Yifan Yang 1 , Doudou Huang 4 , Guanghao Su 5 , Xu Fu 6 , Qingliang Zhao 4 , Zhenqing Hou 1
Affiliation
|
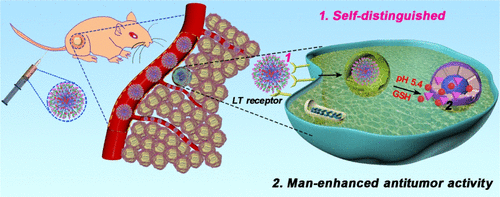
On-demand drug release nanoplatforms are promising alternative strategies for enhancing the therapeutic effect of cancer chemotherapy. However, these nanoplatforms still have many drawbacks including rapid blood clearance, nontargeted specificity, and a lack of immune escape function. Even worse, they are also hindered via the dosage-limiting toxicity of traditional chemotherapeutic drugs. Herein, both dual-functional mannose (enhances the antitumor activity of chemotherapeutic drugs and exhibits an innate affinity against the lectin receptor) and amphiphilic d-α-tocopheryl polyethylene glycol 1000 succinate were selected to be covalently linked via a redox-responsive monothioether linkage. The synthesized self-distinguished polymer (TSM), as a structural motif, can be self-assembled into nanoparticles (TSM NPs) in an aqueous solution, in which doxorubicin (DOX) is loaded by weak interactions (TSM–DOX NPs). These TSM–DOX NPs can provide targeted, on-demand drug release under dual stimuli from lysosomal acidity and glutathione (GSH). In addition, TSM–DOX NPs can be self-distinguished via tumor cells in vitro and specifically self-distinguished from the tumor site in vivo. Further in vitro and in vivo research consistently demonstrated that TSM–DOX NPs display highly synergistic chemotherapeutic effects. Taken together, the data show that the self-distinguished GSH-responsive polymer TSM has the potential to load various therapeutic agents.
中文翻译:

用于细胞内按需药物释放的新型,自识别,双重刺激响应性纳米治疗平台。
按需药物释放纳米平台是增强癌症化学疗法治疗效果的有前途的替代策略。然而,这些纳米平台仍然具有许多缺点,包括血液快速清除,非靶向特异性和缺乏免疫逃逸功能。更糟的是,他们也阻碍了通过传统的化疗药物剂量限制性毒性。本文中,选择双功能甘露糖(增强化疗药物的抗肿瘤活性并表现出对血凝素受体的先天亲和力)和两亲性d -α-生育酚聚乙二醇1000琥珀酸酯通过以下方式共价连接:氧化还原反应性单硫醚键。合成的自区别聚合物(TSM)作为结构基序,可以在水溶液中自组装成纳米粒子(TSM NPs),其中阿霉素(DOX)通过弱相互作用(TSM–DOX NPs)负载。这些TSM-DOX NP可以在溶酶体酸度和谷胱甘肽(GSH)的双重刺激下提供有针对性的按需药物释放。另外,TSM-DOX NPs可以在体外通过肿瘤细胞自识别,而在体内则可以与肿瘤部位自识别。进一步的体外和体内研究一致证明,TSM-DOX NPs具有高度协同的化学治疗作用。综上所述,数据表明,自区别的GSH反应性聚合物TSM具有加载各种治疗剂的潜力。
更新日期:2020-07-06
中文翻译:

用于细胞内按需药物释放的新型,自识别,双重刺激响应性纳米治疗平台。
按需药物释放纳米平台是增强癌症化学疗法治疗效果的有前途的替代策略。然而,这些纳米平台仍然具有许多缺点,包括血液快速清除,非靶向特异性和缺乏免疫逃逸功能。更糟的是,他们也阻碍了通过传统的化疗药物剂量限制性毒性。本文中,选择双功能甘露糖(增强化疗药物的抗肿瘤活性并表现出对血凝素受体的先天亲和力)和两亲性d -α-生育酚聚乙二醇1000琥珀酸酯通过以下方式共价连接:氧化还原反应性单硫醚键。合成的自区别聚合物(TSM)作为结构基序,可以在水溶液中自组装成纳米粒子(TSM NPs),其中阿霉素(DOX)通过弱相互作用(TSM–DOX NPs)负载。这些TSM-DOX NP可以在溶酶体酸度和谷胱甘肽(GSH)的双重刺激下提供有针对性的按需药物释放。另外,TSM-DOX NPs可以在体外通过肿瘤细胞自识别,而在体内则可以与肿瘤部位自识别。进一步的体外和体内研究一致证明,TSM-DOX NPs具有高度协同的化学治疗作用。综上所述,数据表明,自区别的GSH反应性聚合物TSM具有加载各种治疗剂的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号