当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Monitoring Protein-Protein Interactions in the Cyanobacterial Circadian Clock in Real Time via Electron Paramagnetic Resonance Spectroscopy.
Biochemistry ( IF 2.9 ) Pub Date : 2020-05-26 , DOI: 10.1021/acs.biochem.0c00279 Gary K Chow 1 , Archana G Chavan , Joel C Heisler , Yong-Gang Chang , Andy LiWang 2 , R David Britt 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-05-26 , DOI: 10.1021/acs.biochem.0c00279 Gary K Chow 1 , Archana G Chavan , Joel C Heisler , Yong-Gang Chang , Andy LiWang 2 , R David Britt 1
Affiliation
|
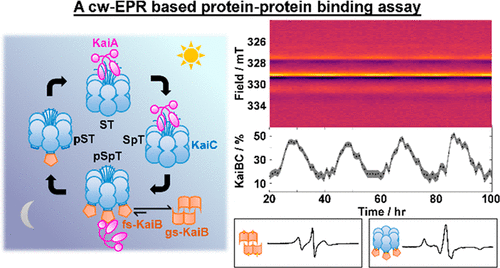
The cyanobacterial circadian clock in Synechococcus elongatus consists of three proteins, KaiA, KaiB, and KaiC. KaiA and KaiB rhythmically interact with KaiC to generate stable oscillations of KaiC phosphorylation with a period of 24 h. The observation of stable circadian oscillations when the three clock proteins are reconstituted and combined in vitro makes it an ideal system for understanding its underlying molecular mechanisms and circadian clocks in general. These oscillations were historically monitored in vitro by gel electrophoresis of reaction mixtures based on the differing electrophoretic mobilities between various phosphostates of KaiC. As the KaiC phospho-distribution represents only one facet of the oscillations, orthogonal tools are necessary to explore other interactions to generate a full description of the system. However, previous biochemical assays are discontinuous or qualitative. To circumvent these limitations, we developed a spin-labeled KaiB mutant that can differentiate KaiC-bound KaiB from free KaiB using continuous-wave electron paramagnetic resonance spectroscopy that is minimally sensitive to KaiA. Similar to wild-type (WT-KaiB), this labeled mutant, in combination with KaiA, sustains robust circadian rhythms of KaiC phosphorylation. This labeled mutant is hence a functional surrogate of WT-KaiB and thus participates in and reports on autonomous macroscopic circadian rhythms generated by mixtures that include KaiA, KaiC, and ATP. Quantitative kinetics could be extracted with improved precision and time resolution. We describe design principles, data analysis, and limitations of this quantitative binding assay and discuss future research necessary to overcome these challenges.
中文翻译:

通过电子顺磁共振波谱实时监测蓝藻生物钟中的蛋白质-蛋白质相互作用。
细长突触球菌中的蓝藻生物钟由KaiA,KaiB和KaiC三种蛋白质组成。KaiA和KaiB与KaiC有节奏地相互作用,在24小时内产生稳定的KaiC磷酸化振荡。三种钟蛋白在体外重组并结合后,观察到稳定的昼夜节律振荡,使其成为理想的系统,可大致了解其潜在的分子机制和昼夜节律。历史上,这些振荡是根据KaiK各种磷酸酯之间的电泳迁移率不同,通过反应混合物的凝胶电泳进行体外监测的。由于KaiC磷酸分布仅表示振荡的一个方面,因此需要正交工具来探索其他相互作用以生成系统的完整描述。然而,先前的生化测定是不连续的或定性的。为了克服这些限制,我们开发了一种自旋标记的KaiB突变体,该突变体可以使用对KaiA最低敏感的连续波电子顺磁共振波谱,将与KaiC结合的KaiB与游离的KaiB区别开来。与野生型(WT-KaiB)相似,此标记的突变体与KaiA结合可维持KaiC磷酸化的强劲生物钟节律。因此,该标记的突变体是WT-KaiB的功能替代物,因此参与并报告了由包括KaiA,KaiC和ATP的混合物产生的自主宏观昼夜节律。可以以改进的精度和时间分辨率提取定量动力学。我们描述了设计原理,数据分析和这种定量结合测定法的局限性,并讨论了克服这些挑战所必需的未来研究。我们开发了一种自旋标记的KaiB突变体,该突变体可以使用对KaiA最低敏感的连续波电子顺磁共振波谱,将KaiC结合的KaiB与游离的KaiB区分开。与野生型(WT-KaiB)相似,此标记的突变体与KaiA结合可维持KaiC磷酸化的强劲生物钟节律。因此,该标记的突变体是WT-KaiB的功能替代物,因此参与并报告了由包括KaiA,KaiC和ATP的混合物产生的自主宏观昼夜节律。可以以改进的精度和时间分辨率提取定量动力学。我们描述了设计原理,数据分析和这种定量结合测定法的局限性,并讨论了克服这些挑战所必需的未来研究。我们开发了一种自旋标记的KaiB突变体,该突变体可以使用对KaiA最低敏感的连续波电子顺磁共振波谱,将与KaiC结合的KaiB与游离的KaiB区别开来。与野生型(WT-KaiB)相似,此标记的突变体与KaiA结合可维持KaiC磷酸化的强劲生物钟节律。因此,该标记的突变体是WT-KaiB的功能替代物,因此参与并报告了由包括KaiA,KaiC和ATP的混合物产生的自主宏观昼夜节律。可以以改进的精度和时间分辨率提取定量动力学。我们描述了设计原理,数据分析和这种定量结合测定法的局限性,并讨论了克服这些挑战所必需的未来研究。
更新日期:2020-07-07
中文翻译:

通过电子顺磁共振波谱实时监测蓝藻生物钟中的蛋白质-蛋白质相互作用。
细长突触球菌中的蓝藻生物钟由KaiA,KaiB和KaiC三种蛋白质组成。KaiA和KaiB与KaiC有节奏地相互作用,在24小时内产生稳定的KaiC磷酸化振荡。三种钟蛋白在体外重组并结合后,观察到稳定的昼夜节律振荡,使其成为理想的系统,可大致了解其潜在的分子机制和昼夜节律。历史上,这些振荡是根据KaiK各种磷酸酯之间的电泳迁移率不同,通过反应混合物的凝胶电泳进行体外监测的。由于KaiC磷酸分布仅表示振荡的一个方面,因此需要正交工具来探索其他相互作用以生成系统的完整描述。然而,先前的生化测定是不连续的或定性的。为了克服这些限制,我们开发了一种自旋标记的KaiB突变体,该突变体可以使用对KaiA最低敏感的连续波电子顺磁共振波谱,将与KaiC结合的KaiB与游离的KaiB区别开来。与野生型(WT-KaiB)相似,此标记的突变体与KaiA结合可维持KaiC磷酸化的强劲生物钟节律。因此,该标记的突变体是WT-KaiB的功能替代物,因此参与并报告了由包括KaiA,KaiC和ATP的混合物产生的自主宏观昼夜节律。可以以改进的精度和时间分辨率提取定量动力学。我们描述了设计原理,数据分析和这种定量结合测定法的局限性,并讨论了克服这些挑战所必需的未来研究。我们开发了一种自旋标记的KaiB突变体,该突变体可以使用对KaiA最低敏感的连续波电子顺磁共振波谱,将KaiC结合的KaiB与游离的KaiB区分开。与野生型(WT-KaiB)相似,此标记的突变体与KaiA结合可维持KaiC磷酸化的强劲生物钟节律。因此,该标记的突变体是WT-KaiB的功能替代物,因此参与并报告了由包括KaiA,KaiC和ATP的混合物产生的自主宏观昼夜节律。可以以改进的精度和时间分辨率提取定量动力学。我们描述了设计原理,数据分析和这种定量结合测定法的局限性,并讨论了克服这些挑战所必需的未来研究。我们开发了一种自旋标记的KaiB突变体,该突变体可以使用对KaiA最低敏感的连续波电子顺磁共振波谱,将与KaiC结合的KaiB与游离的KaiB区别开来。与野生型(WT-KaiB)相似,此标记的突变体与KaiA结合可维持KaiC磷酸化的强劲生物钟节律。因此,该标记的突变体是WT-KaiB的功能替代物,因此参与并报告了由包括KaiA,KaiC和ATP的混合物产生的自主宏观昼夜节律。可以以改进的精度和时间分辨率提取定量动力学。我们描述了设计原理,数据分析和这种定量结合测定法的局限性,并讨论了克服这些挑战所必需的未来研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号