Molecular Cell ( IF 16.0 ) Pub Date : 2020-05-27 , DOI: 10.1016/j.molcel.2020.04.037 Enrico Monachino 1 , Slobodan Jergic 2 , Jacob S Lewis 2 , Zhi-Qiang Xu 2 , Allen T Y Lo 2 , Valerie L O'Shea 3 , James M Berger 3 , Nicholas E Dixon 2 , Antoine M van Oijen 2
|
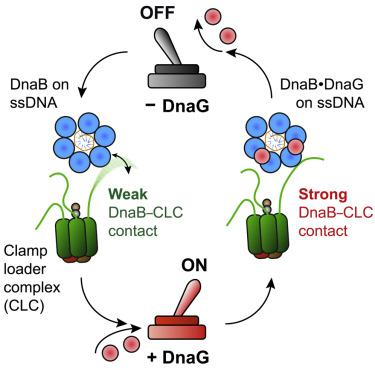
Recent studies of bacterial DNA replication have led to a picture of the replisome as an entity that freely exchanges DNA polymerases and displays intermittent coupling between the helicase and polymerase(s). Challenging the textbook model of the polymerase holoenzyme acting as a stable complex coordinating the replisome, these observations suggest a role of the helicase as the central organizing hub. We show here that the molecular origin of this newly found plasticity lies in the 500-fold increase in strength of the interaction between the polymerase holoenzyme and the replicative helicase upon association of the primase with the replisome. By combining in vitro ensemble-averaged and single-molecule assays, we demonstrate that this conformational switch operates during replication and promotes recruitment of multiple holoenzymes at the fork. Our observations provide a molecular mechanism for polymerase exchange and offer a revised model for the replication reaction that emphasizes its stochasticity.
中文翻译:

酶诱导的构象开关控制细菌复制体的稳定性。
细菌DNA复制的最新研究已使复制体成为可自由交换DNA聚合酶并显示解旋酶和聚合酶之间间歇性偶联的实体的图片。这些发现挑战了聚合酶全酶作为协调复制体的稳定复合体的教科书模型,这些观察结果表明解旋酶作为中央组织中心的作用。我们在这里显示,这种新发现的可塑性的分子起源在于,在将酶与复制酶结合后,聚合酶全酶和复制解旋酶之间相互作用的强度提高了500倍。通过体外结合集合平均法和单分子测定法,我们证明了这种构象转换在复制过程中起作用,并在叉处促进多种全酶的募集。我们的观察结果提供了聚合酶交换的分子机制,并提供了强调其随机性的复制反应模型。


























 京公网安备 11010802027423号
京公网安备 11010802027423号