当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
TFA/TBHP-promoted oxidative cyclisation for the construction of tetracyclic quinazolinones and rutaecarpine
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-05-26 , DOI: 10.1039/d0qo00345j Feng-Cheng Jia 1, 2, 3, 4 , Tian-Zhi Chen 1, 2, 3, 4 , Xiao-Qiang Hu 4, 5, 6, 7, 8
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-05-26 , DOI: 10.1039/d0qo00345j Feng-Cheng Jia 1, 2, 3, 4 , Tian-Zhi Chen 1, 2, 3, 4 , Xiao-Qiang Hu 4, 5, 6, 7, 8
Affiliation
|
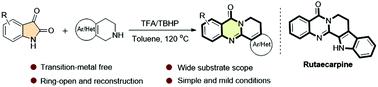
An efficient TFA/TBHP-promoted oxidative cyclisation of readily available isatins with 1,2,3,4-tetrahydroisoquinolines has been firstly developed. This protocol features simple operation, metal-free conditions and broad substrate scope. Under mild conditions, a wide range of tetracyclic quinazolinones were obtained in a highly efficient manner. Moreover, the potential utility of this strategy was demonstrated by one-step synthesis of a natural alkaloid Rutaecarpin.
中文翻译:

TFA / TBHP促进的氧化环化反应,用于构建四环喹唑啉酮和芸香果碱
首先开发了一种高效的TFA / TBHP促进易获得的靛红与1,2,3,4-四氢异喹啉的氧化环化反应。该协议具有操作简单,无金属的条件和广泛的基材范围。在温和的条件下,可以高效地获得各种四环喹唑啉酮。此外,通过天然生物碱果胶的一步合成证明了该策略的潜在效用。
更新日期:2020-06-30
中文翻译:

TFA / TBHP促进的氧化环化反应,用于构建四环喹唑啉酮和芸香果碱
首先开发了一种高效的TFA / TBHP促进易获得的靛红与1,2,3,4-四氢异喹啉的氧化环化反应。该协议具有操作简单,无金属的条件和广泛的基材范围。在温和的条件下,可以高效地获得各种四环喹唑啉酮。此外,通过天然生物碱果胶的一步合成证明了该策略的潜在效用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号