当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
2H‐Indazolo[2,1‐b]phthalazine‐trione derivatives: Inhibition on some metabolic enzymes and molecular docking studies
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-05-25 , DOI: 10.1002/jhet.4019 Parham Taslimi 1 , Fikret Türkan 2 , Kadir Turhan 3 , Halide Sedef Karaman 4 , Zuhal Turgut 3 , İlhami Gulcin 4
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-05-25 , DOI: 10.1002/jhet.4019 Parham Taslimi 1 , Fikret Türkan 2 , Kadir Turhan 3 , Halide Sedef Karaman 4 , Zuhal Turgut 3 , İlhami Gulcin 4
Affiliation
|
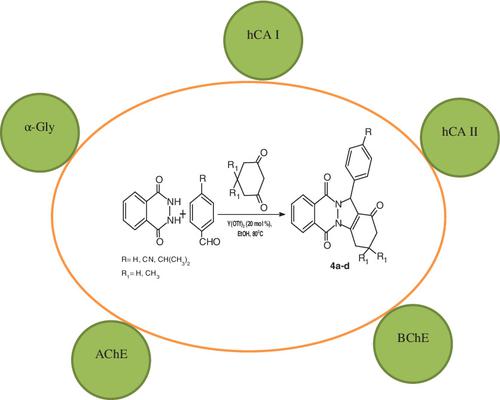
In this study, substituted 2H‐indazolo[2,1‐b ]phthalazine‐1,6,11‐trione compounds (4a–d ) obtained via one‐pot three‐component condensation reaction of aromatic aldehydes, cyclic 1,3‐dione, and phthalhydrazide in ethanol catalyzed by Y(OTf)3 showed satisfactory inhibitory effects against some important enzymes. Also, these molecules had Ki values in the row of 185.92 ± 36.03‐294.82 ± 50.76 nM vs carbonic anhydrase I (CA I), 204.93 ± 46.90‐374.10 ± 83.63 nM against human CA II, 937.16 ± 205.82‐1021.83 ± 193.66 nM against α‐glycosidase (α‐Gly), respectively. For cholinesterase enzymes, the Ki values were found in the range of 47.26 ± 9.62‐72.05 ± 19.47 nM against acetylcholinesterase (AChE) and 65.03 ± 9.88‐102.83 ± 25.04 nM against butyrylcholinesterase (BChE), respectively. The inhibition effects of these compounds against enzymes whose name are AChE, BChE, α‐Gly, hCA I, and hCA II, were compared with control molecules like tacrine, acarbose, and acetazolamide.
中文翻译:

2H-吲哚并[2,1-b]酞嗪-三酮衍生物:对某些代谢酶的抑制作用和分子对接研究
在这项研究中,取代的2H-吲哚并[ 2,1- b ]酞嗪-1,6,11-三酮化合物(4a-d)是通过芳香醛,环状1,3-二酮的一锅三组分缩合反应获得的,Y(OTf)3催化乙醇中的邻苯二甲酰肼显示出对某些重要酶的令人满意的抑制作用。同样,这些分子相对于碳酸酐酶I(CA I)的行中的K i值为185.92±36.03-294.82±50.76 nM,针对人CA II的204.93±46.90-374.10±83.63 nM,937.16±205.82-11021.83±193.66 nM分别针对α-糖苷酶(α-Gly)。对于胆碱酯酶,K i抗乙酰胆碱酯酶(AChE)的值分别为47.26±9.62-72.05±19.47 nM和抗丁酰胆碱酯酶(BChE)的范围为65.03±9.88-102.83±25.04 nM。比较了这些化合物对名称为AChE,BChE,α-Gly,hCA I和hCA II的酶的抑制作用,并将其与他克林,阿卡波糖和乙酰唑胺等对照分子进行了比较。
更新日期:2020-05-25
中文翻译:

2H-吲哚并[2,1-b]酞嗪-三酮衍生物:对某些代谢酶的抑制作用和分子对接研究
在这项研究中,取代的2H-吲哚并[ 2,1- b ]酞嗪-1,6,11-三酮化合物(4a-d)是通过芳香醛,环状1,3-二酮的一锅三组分缩合反应获得的,Y(OTf)3催化乙醇中的邻苯二甲酰肼显示出对某些重要酶的令人满意的抑制作用。同样,这些分子相对于碳酸酐酶I(CA I)的行中的K i值为185.92±36.03-294.82±50.76 nM,针对人CA II的204.93±46.90-374.10±83.63 nM,937.16±205.82-11021.83±193.66 nM分别针对α-糖苷酶(α-Gly)。对于胆碱酯酶,K i抗乙酰胆碱酯酶(AChE)的值分别为47.26±9.62-72.05±19.47 nM和抗丁酰胆碱酯酶(BChE)的范围为65.03±9.88-102.83±25.04 nM。比较了这些化合物对名称为AChE,BChE,α-Gly,hCA I和hCA II的酶的抑制作用,并将其与他克林,阿卡波糖和乙酰唑胺等对照分子进行了比较。



























 京公网安备 11010802027423号
京公网安备 11010802027423号