当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The role of HSP90 molecular chaperones in hepatocellular carcinoma.
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2020-05-26 , DOI: 10.1002/jcp.29776 Masoud Nouri-Vaskeh 1, 2 , Leila Alizadeh 3 , Khalil Hajiasgharzadeh 1 , Ahad Mokhtarzadeh 1 , Monireh Halimi 4 , Behzad Baradaran 1, 5
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2020-05-26 , DOI: 10.1002/jcp.29776 Masoud Nouri-Vaskeh 1, 2 , Leila Alizadeh 3 , Khalil Hajiasgharzadeh 1 , Ahad Mokhtarzadeh 1 , Monireh Halimi 4 , Behzad Baradaran 1, 5
Affiliation
|
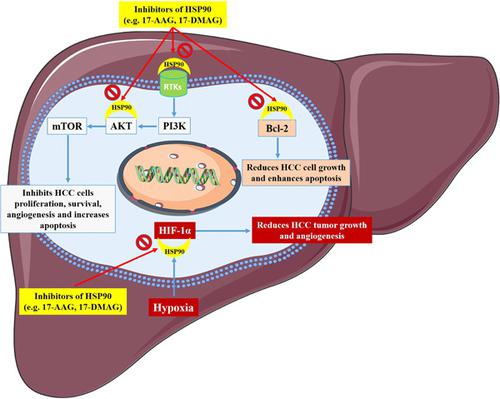
Misfolded proteins have enhanced formation of toxic oligomers and nonfunctional protein copies lead to recruiting wild‐type protein types. Heat shock protein 90 (HSP90) is a molecular chaperone generated by cells that are involved in many cellular functions through regulation of folding and/or localization of large multi‐protein complexes as well as client proteins. HSP90 can regulate a number of different cellular processes including cell proliferation, motility, angiogenesis, signal transduction, and adaptation to stress. HSP90 makes the mutated oncoproteins able to avoid misfolding and degradation and permits the malignant transformation. As a result, HSP90 is an important factor in several signaling pathways associated with tumorigenicity, therapy resistance, and inhibiting apoptosis. Clinically, the upregulation of HSP90 expression in hepatocellular carcinoma (HCC) is linked with advanced stages and inappropriate survival in cases suffering from this kind of cancer. The present review comprehensively assesses HSP90 functions and its possible usefulness as a potential diagnostic biomarker and therapeutic option for HCC.
中文翻译:

HSP90分子伴侣在肝细胞癌中的作用。
折叠错误的蛋白质会增强毒性低聚物的形成,非功能性蛋白质拷贝会导致募集野生型蛋白质。热休克蛋白90(HSP90)是由细胞产生的分子伴侣,通过调节大型多蛋白复合物以及客户蛋白的折叠和/或定位来参与许多细胞功能。HSP90可以调节许多不同的细胞过程,包括细胞增殖,运动,血管生成,信号转导和适应压力。HSP90使突变的癌蛋白能够避免错误折叠和降解,并允许恶性转化。结果,HSP90是与致瘤性,治疗抗性和抑制细胞凋亡相关的几种信号通路中的重要因素。临床上 肝细胞癌(HCC)中HSP90表达的上调与晚期阶段以及在罹患这种癌症的患者中的不适当生存有关。本文对HSP90的功能及其作为HCC潜在的诊断生物标志物和治疗选择的可能用途进行了全面评估。
更新日期:2020-05-26
中文翻译:

HSP90分子伴侣在肝细胞癌中的作用。
折叠错误的蛋白质会增强毒性低聚物的形成,非功能性蛋白质拷贝会导致募集野生型蛋白质。热休克蛋白90(HSP90)是由细胞产生的分子伴侣,通过调节大型多蛋白复合物以及客户蛋白的折叠和/或定位来参与许多细胞功能。HSP90可以调节许多不同的细胞过程,包括细胞增殖,运动,血管生成,信号转导和适应压力。HSP90使突变的癌蛋白能够避免错误折叠和降解,并允许恶性转化。结果,HSP90是与致瘤性,治疗抗性和抑制细胞凋亡相关的几种信号通路中的重要因素。临床上 肝细胞癌(HCC)中HSP90表达的上调与晚期阶段以及在罹患这种癌症的患者中的不适当生存有关。本文对HSP90的功能及其作为HCC潜在的诊断生物标志物和治疗选择的可能用途进行了全面评估。


























 京公网安备 11010802027423号
京公网安备 11010802027423号