Structure ( IF 5.7 ) Pub Date : 2020-05-26 , DOI: 10.1016/j.str.2020.05.002 Yibin Xu 1 , Nicholas S Kirk 1 , Hariprasad Venugopal 2 , Mai B Margetts 3 , Tristan I Croll 4 , Jarrod J Sandow 1 , Andrew I Webb 1 , Carlie A Delaine 5 , Briony E Forbes 5 , Michael C Lawrence 1
|
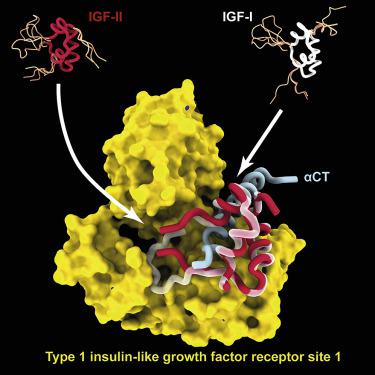
Human type 1 insulin-like growth factor receptor (IGF-1R) signals chiefly in response to the binding of insulin-like growth factor I. Relatively little is known about the role of insulin-like growth factor II signaling via IGF-1R, despite the affinity of insulin-like growth factor II for IGF-1R being within an order of magnitude of that of insulin-like growth factor I. Here, we describe the cryoelectron microscopy structure of insulin-like growth factor II bound to a leucine-zipper-stabilized IGF-1R ectodomain, determined in two conformations to a maximum average resolution of 3.2 Å. The two conformations differ in the relative separation of their respective points of membrane entry, and comparison with the structure of insulin-like growth factor I bound to IGF-1R reveals long-suspected differences in the way in which the critical C domain of the respective growth factors interact with IGF-1R.
中文翻译:

IGF-II如何与人类1型胰岛素样生长因子受体结合。
人1型胰岛素样生长因子受体(IGF-1R)的信号主要是响应于胰岛素样生长因子I的结合。尽管有人知道,通过IGF-1R进行的胰岛素样生长因子II信号的作用知之甚少胰岛素样生长因子II对IGF-1R的亲和力在胰岛素样生长因子I亲和力的数量级之内。在这里,我们描述胰岛素样生长因子II与亮氨酸拉链结合的冷冻电子显微镜结构稳定的IGF-1R胞外域,以两个构象确定,最大平均分辨率为3.2。两种构型在各自的膜进入点的相对分离方面有所不同,



























 京公网安备 11010802027423号
京公网安备 11010802027423号