Cell Stem Cell ( IF 23.9 ) Pub Date : 2020-05-26 , DOI: 10.1016/j.stem.2020.05.004 Elisa Giacomelli 1 , Viviana Meraviglia 1 , Giulia Campostrini 1 , Amy Cochrane 1 , Xu Cao 1 , Ruben W J van Helden 1 , Ana Krotenberg Garcia 1 , Maria Mircea 2 , Sarantos Kostidis 3 , Richard P Davis 1 , Berend J van Meer 1 , Carolina R Jost 4 , Abraham J Koster 4 , Hailiang Mei 5 , David G Míguez 6 , Aat A Mulder 4 , Mario Ledesma-Terrón 6 , Giulio Pompilio 7 , Luca Sala 1 , Daniela C F Salvatori 8 , Roderick C Slieker 9 , Elena Sommariva 10 , Antoine A F de Vries 11 , Martin Giera 3 , Stefan Semrau 2 , Leon G J Tertoolen 1 , Valeria V Orlova 1 , Milena Bellin 12 , Christine L Mummery 13
|
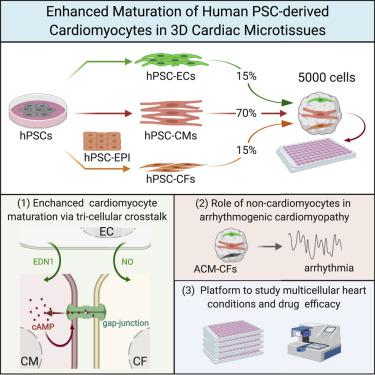
Cardiomyocytes (CMs) from human induced pluripotent stem cells (hiPSCs) are functionally immature, but this is improved by incorporation into engineered tissues or forced contraction. Here, we showed that tri-cellular combinations of hiPSC-derived CMs, cardiac fibroblasts (CFs), and cardiac endothelial cells also enhance maturation in easily constructed, scaffold-free, three-dimensional microtissues (MTs). hiPSC-CMs in MTs with CFs showed improved sarcomeric structures with T-tubules, enhanced contractility, and mitochondrial respiration and were electrophysiologically more mature than MTs without CFs. Interactions mediating maturation included coupling between hiPSC-CMs and CFs through connexin 43 (CX43) gap junctions and increased intracellular cyclic AMP (cAMP). Scaled production of thousands of hiPSC-MTs was highly reproducible across lines and differentiated cell batches. MTs containing healthy-control hiPSC-CMs but hiPSC-CFs from patients with arrhythmogenic cardiomyopathy strikingly recapitulated features of the disease. Our MT model is thus a simple and versatile platform for modeling multicellular cardiac diseases that will facilitate industry and academic engagement in high-throughput molecular screening.
中文翻译:

人iPSC衍生的心脏基质细胞增强了3D心脏微组织的成熟度,并揭示了非心肌细胞对心脏病的贡献。
来自人诱导的多能干细胞(hiPSC)的心肌细胞(CM)在功能上不成熟,但这可以通过整合入工程组织或强制收缩而得到改善。在这里,我们表明,hiPSC衍生的CM,心脏成纤维细胞(CF)和心脏内皮细胞的三细胞组合也增强了易于构建,无支架的三维显微组织(MT)的成熟度。具有CFs的MTs中的hiPSC-CMs显示具有T管的肌节结构改善,收缩力增强和线粒体呼吸,并且在电生理上比不含CFs的MTs更成熟。介导成熟的相互作用包括hiPSC-CM和CF之间通过连接蛋白43(CX43)间隙连接和细胞内环状AMP(cAMP)的偶联。跨系和分化的细胞批次可高度重现数千个hiPSC-MT的大规模生产。含有健康对照hiPSC-CM但来自心律失常性心肌病患者的hiPSC-CF的MT明显概括了该疾病的特征。因此,我们的MT模型是用于建模多细胞心脏病的简单而通用的平台,这将有助于行业和学术界参与高通量分子筛查。



























 京公网安备 11010802027423号
京公网安备 11010802027423号