当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrocatalytic three-component annulation-halosulfonylation of 1,6-enynes toward 1-indanones using sodium halides as both halogen sources and electrolytes
Green Chemistry ( IF 9.8 ) Pub Date : 2020-05-25 , DOI: 10.1039/d0gc00771d Tian-Shu Zhang 1, 2, 3, 4, 5 , Wen-Juan Hao 1, 2, 3, 4, 5 , Rong Wang 1, 2, 3, 4, 5 , Shi-Chao Wang 1, 2, 3, 4, 5 , Shu-Jiang Tu 1, 2, 3, 4, 5 , Bo Jiang 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2020-05-25 , DOI: 10.1039/d0gc00771d Tian-Shu Zhang 1, 2, 3, 4, 5 , Wen-Juan Hao 1, 2, 3, 4, 5 , Rong Wang 1, 2, 3, 4, 5 , Shi-Chao Wang 1, 2, 3, 4, 5 , Shu-Jiang Tu 1, 2, 3, 4, 5 , Bo Jiang 1, 2, 3, 4, 5
Affiliation
|
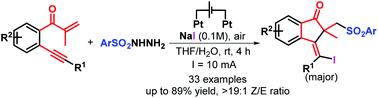
A new electrochemically induced three-component annulation-halosulfonylation of 1,6-enynes has been developed for stereoselective synthesis of 33 examples of 1-indanones with generally good yields under environmentally benign conditions. This electrochemical strategy can proceed in a simple undivided cell, avoiding any transition metal catalysts, chemical oxidants and additives. This reaction tolerates a wide scope of substrates, which provides a new and green access to fabricate important bioactive 1-indanone scaffolds. Notably, readily available and low-cost halogen salts play triple roles of an electrolyte and a redox catalyst as well as a halogenating reagent.
中文翻译:

使用卤化钠作为卤素源和电解质,将1,6-烯炔向1-茚满酮电催化三组分环化-磺酰化
已经开发了一种新的电化学诱导的1,6-炔烃的三组分环化-磺酰化,可在环境良性条件下立体选择性地合成33个1-茚满酮实例。这种电化学策略可以在简单的不分隔电池中进行,避免使用任何过渡金属催化剂,化学氧化剂和添加剂。该反应可耐受各种各样的底物,这为制造重要的生物活性1-茚满酮骨架提供了新的途径。值得注意的是,易于获得且低成本的卤素盐在电解质和氧化还原催化剂以及卤化试剂中起着三重作用。
更新日期:2020-07-06
中文翻译:

使用卤化钠作为卤素源和电解质,将1,6-烯炔向1-茚满酮电催化三组分环化-磺酰化
已经开发了一种新的电化学诱导的1,6-炔烃的三组分环化-磺酰化,可在环境良性条件下立体选择性地合成33个1-茚满酮实例。这种电化学策略可以在简单的不分隔电池中进行,避免使用任何过渡金属催化剂,化学氧化剂和添加剂。该反应可耐受各种各样的底物,这为制造重要的生物活性1-茚满酮骨架提供了新的途径。值得注意的是,易于获得且低成本的卤素盐在电解质和氧化还原催化剂以及卤化试剂中起着三重作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号