当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
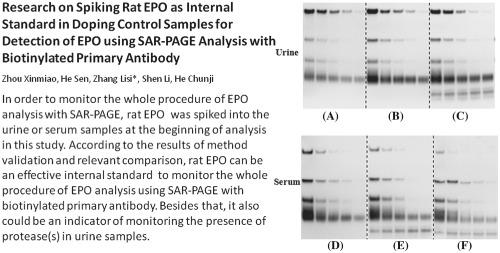
Research on spiking rat EPO as internal standard in doping control samples for detection of EPO using SAR-PAGE analysis with biotinylated primary antibody.
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2020-06-24 , DOI: 10.1002/dta.2863 Xinmiao Zhou 1 , Sen He 1 , Lisi Zhang 1 , Li Shen 1 , Chunji He 1
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2020-06-24 , DOI: 10.1002/dta.2863 Xinmiao Zhou 1 , Sen He 1 , Lisi Zhang 1 , Li Shen 1 , Chunji He 1
Affiliation
|
According to the current Technical Document (TD) for erythropoietin (EPO), SAR‐PAGE is the most commonly applied method for both screening and confirmation procedures. Although this method is effective and robust, it lacks an internal standard (IS) to monitor the efficiency of analysis for each sample covering every step of the whole procedure, including preparation, immunopurification, and western blotting. This internal standard needs to be recognized by both anti‐EPO antibodies used for immunopurification and western blotting, respectively. Besides that, the band of IS could not be allowed to interfere with the recognition of all types of targeted EPO and analogs. To meet these two principles, rat EPO was selected. In this study, rat EPO was used to spike both urine and blood samples at the beginning of analysis. After preparation and immunopurification, single blotting was performed with biotinylated AE7A5 as the primary antibody, followed by incubation with streptavidin‐coupled HRP. Based on the comparison of different immunopurification methods, the AB‐286‐NA antibody coupled to M‐280 magnetic beads was the better choice for urine samples, whereas the MAIIA column was suitable for blood samples. All these methods were validated for selectivity, repeatability, and sensitivity. The modified method in this study could not only eliminate the cross‐reactivity between antibodies but also monitor the whole procedure of the analysis of EPO with spiked rat EPO. Besides that, rat EPO could also be used as an indicator for monitoring the presence of protease(s) in urine samples.
中文翻译:

使用生物素化一抗的SAR-PAGE分析在掺杂对照样品中掺入大鼠EPO作为内标以检测EPO的研究。
根据当前的促红细胞生成素(EPO)技术文件(TD),SAR-PAGE是筛查和确认过程中最常用的方法。尽管此方法既有效又稳健,但它缺少一个内标(IS)来监控涵盖整个过程的每个步骤(包括制备,免疫纯化和蛋白质印迹)的每个样品的分析效率。分别用于免疫纯化和蛋白质印迹的两种抗EPO抗体都需要识别此内标。除此之外,IS频段不允许干扰所有类型的目标EPO和类似物的识别。为了满足这两个原则,选择了大鼠EPO。在这项研究中,大鼠EPO用于在分析开始时对尿液和血液样品进行加标。制备和免疫纯化后,以生物素化的AE7A5作为一抗进行单印迹,然后与链霉亲和素偶联的HRP孵育。根据不同免疫纯化方法的比较,AB-286-NA抗体与M-280磁珠偶联是尿液样品的更好选择,而MAIIA色谱柱则适合血样。对所有这些方法的选择性,可重复性和敏感性进行了验证。这项研究中的改进方法不仅可以消除抗体之间的交叉反应,而且可以监控加标大鼠EPO的EPO分析的整个过程。除此之外,大鼠EPO还可以用作监测尿液样品中蛋白酶存在的指标。使用生物素化的AE7A5作为一抗进行单印迹,然后与链霉亲和素偶联的HRP一起孵育。根据不同免疫纯化方法的比较,AB-286-NA抗体与M-280磁珠偶联是尿液样品的更好选择,而MAIIA色谱柱则适合血样。对所有这些方法的选择性,可重复性和敏感性进行了验证。这项研究中的改进方法不仅可以消除抗体之间的交叉反应,而且可以监控加标大鼠EPO的EPO分析的整个过程。除此之外,大鼠EPO还可以用作监测尿液样品中蛋白酶存在的指标。使用生物素化的AE7A5作为一抗进行单印迹,然后与链霉亲和素偶联的HRP一起孵育。根据不同免疫纯化方法的比较,AB-286-NA抗体与M-280磁珠偶联是尿液样品的更好选择,而MAIIA色谱柱则适合血样。对所有这些方法的选择性,可重复性和敏感性进行了验证。这项研究中的改进方法不仅可以消除抗体之间的交叉反应,而且可以监控加标大鼠EPO的EPO分析的整个过程。除此之外,大鼠EPO还可以用作监测尿液样品中蛋白酶存在的指标。AB‐286‐NA抗体与M‐280磁珠偶联是尿液样品的更好选择,而MAIIA色谱柱适合于血液样品。对所有这些方法的选择性,可重复性和敏感性进行了验证。这项研究中的改进方法不仅可以消除抗体之间的交叉反应,而且可以监控加标大鼠EPO的EPO分析的整个过程。除此之外,大鼠EPO还可以用作监测尿液样品中蛋白酶存在的指标。AB‐286‐NA抗体与M‐280磁珠偶联是尿液样品的更好选择,而MAIIA色谱柱适合于血液样品。对所有这些方法的选择性,可重复性和敏感性进行了验证。这项研究中的改进方法不仅可以消除抗体之间的交叉反应,而且可以监控加标大鼠EPO的EPO分析的整个过程。除此之外,大鼠EPO还可以用作监测尿液样品中蛋白酶存在的指标。这项研究中的改进方法不仅可以消除抗体之间的交叉反应,而且可以监控加标大鼠EPO的EPO分析的整个过程。除此之外,大鼠EPO还可以用作监测尿液样品中蛋白酶存在的指标。这项研究中的改进方法不仅可以消除抗体之间的交叉反应,而且可以监控加标大鼠EPO的EPO分析的整个过程。除此之外,大鼠EPO还可以用作监测尿液样品中蛋白酶存在的指标。
更新日期:2020-06-24
中文翻译:

使用生物素化一抗的SAR-PAGE分析在掺杂对照样品中掺入大鼠EPO作为内标以检测EPO的研究。
根据当前的促红细胞生成素(EPO)技术文件(TD),SAR-PAGE是筛查和确认过程中最常用的方法。尽管此方法既有效又稳健,但它缺少一个内标(IS)来监控涵盖整个过程的每个步骤(包括制备,免疫纯化和蛋白质印迹)的每个样品的分析效率。分别用于免疫纯化和蛋白质印迹的两种抗EPO抗体都需要识别此内标。除此之外,IS频段不允许干扰所有类型的目标EPO和类似物的识别。为了满足这两个原则,选择了大鼠EPO。在这项研究中,大鼠EPO用于在分析开始时对尿液和血液样品进行加标。制备和免疫纯化后,以生物素化的AE7A5作为一抗进行单印迹,然后与链霉亲和素偶联的HRP孵育。根据不同免疫纯化方法的比较,AB-286-NA抗体与M-280磁珠偶联是尿液样品的更好选择,而MAIIA色谱柱则适合血样。对所有这些方法的选择性,可重复性和敏感性进行了验证。这项研究中的改进方法不仅可以消除抗体之间的交叉反应,而且可以监控加标大鼠EPO的EPO分析的整个过程。除此之外,大鼠EPO还可以用作监测尿液样品中蛋白酶存在的指标。使用生物素化的AE7A5作为一抗进行单印迹,然后与链霉亲和素偶联的HRP一起孵育。根据不同免疫纯化方法的比较,AB-286-NA抗体与M-280磁珠偶联是尿液样品的更好选择,而MAIIA色谱柱则适合血样。对所有这些方法的选择性,可重复性和敏感性进行了验证。这项研究中的改进方法不仅可以消除抗体之间的交叉反应,而且可以监控加标大鼠EPO的EPO分析的整个过程。除此之外,大鼠EPO还可以用作监测尿液样品中蛋白酶存在的指标。使用生物素化的AE7A5作为一抗进行单印迹,然后与链霉亲和素偶联的HRP一起孵育。根据不同免疫纯化方法的比较,AB-286-NA抗体与M-280磁珠偶联是尿液样品的更好选择,而MAIIA色谱柱则适合血样。对所有这些方法的选择性,可重复性和敏感性进行了验证。这项研究中的改进方法不仅可以消除抗体之间的交叉反应,而且可以监控加标大鼠EPO的EPO分析的整个过程。除此之外,大鼠EPO还可以用作监测尿液样品中蛋白酶存在的指标。AB‐286‐NA抗体与M‐280磁珠偶联是尿液样品的更好选择,而MAIIA色谱柱适合于血液样品。对所有这些方法的选择性,可重复性和敏感性进行了验证。这项研究中的改进方法不仅可以消除抗体之间的交叉反应,而且可以监控加标大鼠EPO的EPO分析的整个过程。除此之外,大鼠EPO还可以用作监测尿液样品中蛋白酶存在的指标。AB‐286‐NA抗体与M‐280磁珠偶联是尿液样品的更好选择,而MAIIA色谱柱适合于血液样品。对所有这些方法的选择性,可重复性和敏感性进行了验证。这项研究中的改进方法不仅可以消除抗体之间的交叉反应,而且可以监控加标大鼠EPO的EPO分析的整个过程。除此之外,大鼠EPO还可以用作监测尿液样品中蛋白酶存在的指标。这项研究中的改进方法不仅可以消除抗体之间的交叉反应,而且可以监控加标大鼠EPO的EPO分析的整个过程。除此之外,大鼠EPO还可以用作监测尿液样品中蛋白酶存在的指标。这项研究中的改进方法不仅可以消除抗体之间的交叉反应,而且可以监控加标大鼠EPO的EPO分析的整个过程。除此之外,大鼠EPO还可以用作监测尿液样品中蛋白酶存在的指标。


























 京公网安备 11010802027423号
京公网安备 11010802027423号