当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, spectroscopic, thermal and antimicrobial investigations of new mono and binuclear Cu(II), Co(II), Ni(II), and Zn(II) thiosemicarbazide complexes
Journal of Molecular Structure ( IF 3.8 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.molstruc.2020.128516 Moamen S. Refat , A.A.M. Belal , I.M. El-Deen , N. Hassan , R. Zakaria
Journal of Molecular Structure ( IF 3.8 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.molstruc.2020.128516 Moamen S. Refat , A.A.M. Belal , I.M. El-Deen , N. Hassan , R. Zakaria
|
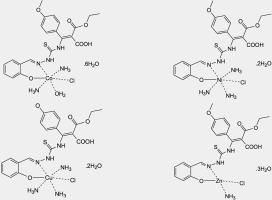
Abstract The thiosemicarbazone (TSC) derivative chelate was synthesized by condensation of 2-hydroxybenzaldehyde with substituted thiosemicarbazide. Eight mono and binuclear transition metal(II) complexes of TSC derivative have been prepared in 1:1 and 2:1 (M:TSC) ratios. Elemental analysis, molar conductivity, magnetic measurements, thermogravimetric, UV–Vis., FTIR, 1H NMR, and mass spectra have been used to identify those complexes. The 1:1 complexes have the general formula [M(TSC)(NH3) x(Cl)y(H2O)z]. nH2O, where M = Ni, Cu and Zn, x = 3, 3 and 1; y = 1; n = 2, 2 and 3 respectively, while Co(II) complex has formula [Co(TSC)(NH3)2(Cl)(H2O)]·6H2O. The binuclear 2:1 complexes have the general formulas [Co2(TSC)(NH3)2(Cl)2(H2O)4]·2H2O, [Ni2(TSC)(NH3)4(Cl)2(H2O)2]·4H2O, [Cu2(TSC)(NH3)4(Cl)2(H2O)2]·2H2O, and [Zn2(TSC)(NH3)2(Cl)2] 2H2O respectively. The mononuclear and binuclear complexes are found to have distorted octahedral stereochemistry in which one of the central metal ions is bonded to TSC chelate through phenolate oxygen atom and azomethine nitrogen atom, while in case of binuclear complexes the other central metal ions is coordinated toward TSC chelate via two oxygen atoms of carboxylate group that associated from the hydrolyses of the terminal cyano group. The TSC ligand and its complexes have been studied for their possible biological activity including antibacterial and antifungal activity.
中文翻译:

新型单核和双核 Cu(II)、Co(II)、Ni(II) 和 Zn(II) 氨基硫脲配合物的合成、光谱、热和抗菌研究
摘要 通过2-羟基苯甲醛与取代氨基硫脲缩合合成缩氨基硫脲(TSC)衍生物螯合物。已经以 1:1 和 2:1 (M:TSC) 的比例制备了八种 TSC 衍生物的单核和双核过渡金属 (II) 配合物。元素分析、摩尔电导率、磁性测量、热重分析、UV-Vis.、FTIR、1H NMR 和质谱已用于鉴定这些复合物。1:1 配合物的通式为 [M(TSC)(NH3) x(Cl)y(H2O)z]。nH2O,其中 M = Ni、Cu 和 Zn,x = 3、3 和 1;y = 1; n 分别为 2、2 和 3,而 Co(II) 配合物的分子式为 [Co(TSC)(NH3)2(Cl)(H2O)]·6H2O。双核 2:1 配合物的通式为 [Co2(TSC)(NH3)2(Cl)2(H2O)4]·2H2O, [Ni2(TSC)(NH3)4(Cl)2(H2O)2]· 4H2O、[Cu2(TSC)(NH3)4(Cl)2(H2O)2]·2H2O 和 [Zn2(TSC)(NH3)2(Cl)2]2H2O。发现单核和双核配合物具有扭曲的八面体立体化学,其中一个中心金属离子通过酚氧原子和偶氮甲碱氮原子与 TSC 螯合物键合,而在双核配合物的情况下,另一个中心金属离子与 TSC 螯合物配位通过羧基的两个氧原子,它们与末端氰基的水解相关联。已经研究了 TSC 配体及其复合物可能的生物活性,包括抗菌和抗真菌活性。而在双核配合物的情况下,其他中心金属离子通过羧基的两个氧原子与 TSC 螯合物配位,这些氧原子与末端氰基的水解有关。已经研究了 TSC 配体及其复合物可能的生物活性,包括抗菌和抗真菌活性。而在双核配合物的情况下,其他中心金属离子通过羧酸根的两个氧原子与 TSC 螯合物配位,这些氧原子与末端氰基的水解有关。已经研究了 TSC 配体及其复合物可能的生物活性,包括抗菌和抗真菌活性。
更新日期:2020-10-01
中文翻译:

新型单核和双核 Cu(II)、Co(II)、Ni(II) 和 Zn(II) 氨基硫脲配合物的合成、光谱、热和抗菌研究
摘要 通过2-羟基苯甲醛与取代氨基硫脲缩合合成缩氨基硫脲(TSC)衍生物螯合物。已经以 1:1 和 2:1 (M:TSC) 的比例制备了八种 TSC 衍生物的单核和双核过渡金属 (II) 配合物。元素分析、摩尔电导率、磁性测量、热重分析、UV-Vis.、FTIR、1H NMR 和质谱已用于鉴定这些复合物。1:1 配合物的通式为 [M(TSC)(NH3) x(Cl)y(H2O)z]。nH2O,其中 M = Ni、Cu 和 Zn,x = 3、3 和 1;y = 1; n 分别为 2、2 和 3,而 Co(II) 配合物的分子式为 [Co(TSC)(NH3)2(Cl)(H2O)]·6H2O。双核 2:1 配合物的通式为 [Co2(TSC)(NH3)2(Cl)2(H2O)4]·2H2O, [Ni2(TSC)(NH3)4(Cl)2(H2O)2]· 4H2O、[Cu2(TSC)(NH3)4(Cl)2(H2O)2]·2H2O 和 [Zn2(TSC)(NH3)2(Cl)2]2H2O。发现单核和双核配合物具有扭曲的八面体立体化学,其中一个中心金属离子通过酚氧原子和偶氮甲碱氮原子与 TSC 螯合物键合,而在双核配合物的情况下,另一个中心金属离子与 TSC 螯合物配位通过羧基的两个氧原子,它们与末端氰基的水解相关联。已经研究了 TSC 配体及其复合物可能的生物活性,包括抗菌和抗真菌活性。而在双核配合物的情况下,其他中心金属离子通过羧基的两个氧原子与 TSC 螯合物配位,这些氧原子与末端氰基的水解有关。已经研究了 TSC 配体及其复合物可能的生物活性,包括抗菌和抗真菌活性。而在双核配合物的情况下,其他中心金属离子通过羧酸根的两个氧原子与 TSC 螯合物配位,这些氧原子与末端氰基的水解有关。已经研究了 TSC 配体及其复合物可能的生物活性,包括抗菌和抗真菌活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号